Black youth face multiple barriers in accessing mental health care, experts say
Black youth in Canada face multiple barriers in getting access to mental health services — and health-care providers can make the situation more difficult, experts say.

Black youth in Canada face multiple barriers in getting access to mental health services — and health-care providers can make the situation more difficult, experts say.

A New Brunswick woman suffering from sarcoidosis, a disease that limits your lung capacity, is in need of a double lung transplant.
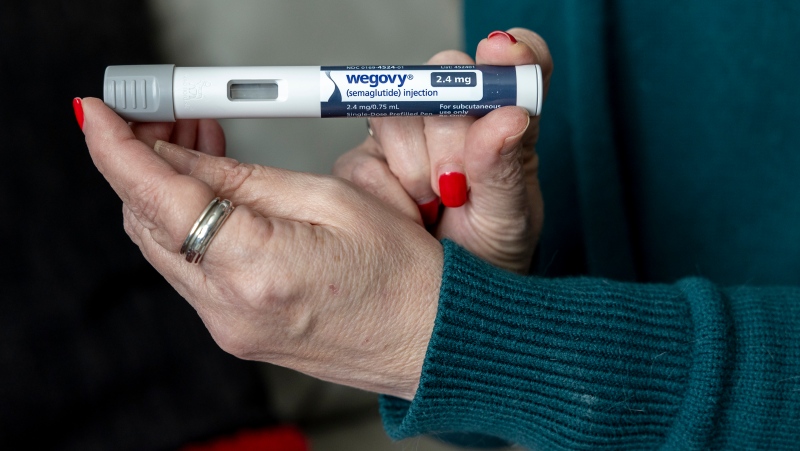
As Wegovy becomes available to Canadians starting Monday, a medical expert is cautioning patients wanting to use the drug to lose weight that no medication is a ''magic bullet,' and the new medication is meant particularly for people who meet certain criteria related to obesity and weight.