
Expert warns of food consumption habits amid rising prices
A new survey by Dalhousie University's Agri-Food Analytics Lab asked Canadians about their food consumption habits amid rising prices.
The U.S. Food and Drug Administration has expanded the emergency use authorization of Regeneron Pharmaceuticals Inc's COVID-19 antibody cocktail, enabling its use as a preventive treatment for the illness in certain people.
The company said the authorization enables the therapy to be used in people exposed to an infected individual, or who are at high risk of exposure to an infected individual in settings such as nursing homes or prisons.
The combo therapy, REGEN-COV, was authorized in November for emergency use to treat people with mild-to-moderate COVID-19 in the United States.
REGEN-COV, a combination of casirivimab and imdevimab, protected household contacts from exposure to SARS-CoV-2, with 72% protection against symptomatic infections in the first week, and 93% after that, according to trial data released by the company in April.
Regeneron said the expanded authorization will help address the needs of immunocompromised people, including those taking immunosuppressive medicines, whose bodies may not mount an adequate response to COVID-19 vaccination.
(Reporting by Mrinalika Roy in Bengaluru; Editing by Devika Syamnath)

A new survey by Dalhousie University's Agri-Food Analytics Lab asked Canadians about their food consumption habits amid rising prices.
Royal commentator Afua Hagan writes that when King Charles recently admitted Catherine to the Order of the Companions of Honour, it not only made history, but it reinforced the strong bond between the King and his beloved daughter-in-law.
Charlie Woods failed to advance in a U.S. Open local qualifying event Thursday, shooting a 9-over 81 at Legacy Golf & Tennis Club.
When it comes to cardiovascular fitness, you may tend to focus on activities that move you forward, such as walking, running and cycling.
Ashley Dickey and her mother rented part of the same Coquitlam duplex in three different decades under three different landlords.
A man who fell into a crevasse while leading a backcountry ski group deep in the Canadian Rockies has died.
MPP Sarah Jama was asked to leave the Legislative Assembly of Ontario by House Speaker Ted Arnott on Thursday for wearing a keffiyeh, a garment which has been banned at Queen’s Park.
After Prime Minister Justin Trudeau said the federal government would still send Canada Carbon Rebate cheques to Saskatchewan residents, despite Saskatchewan Premier Scott Moe's decision to stop collecting the carbon tax on natural gas or home heating, questions were raised about whether other provinces would follow suit. CTV News reached out across the country and here's what we found out.
As Donald Trump was running for president in 2016, his old friend at the National Enquirer was scooping up potentially damaging stories about the candidate and paying out tens of thousands of dollars to keep them from the public eye.
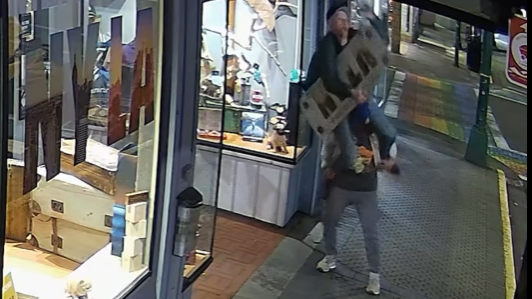
Mounties in Nanaimo, B.C., say two late-night revellers are lucky their allegedly drunken antics weren't reported to police after security cameras captured the men trying to steal a heavy sign from a downtown business.
A property tax bill is perplexing a small townhouse community in Fergus, Ont.
When identical twin sisters Kim and Michelle Krezonoski were invited to compete against some of the world’s most elite female runners at last week’s Boston Marathon, they were in disbelief.
The giant stone statues guarding the Lions Gate Bridge have been dressed in custom Vancouver Canucks jerseys as the NHL playoffs get underway.
A local Oilers fan is hoping to see his team cut through the postseason, so he can cut his hair.
A family from Laval, Que. is looking for answers... and their father's body. He died on vacation in Cuba and authorities sent someone else's body back to Canada.
A former educational assistant is calling attention to the rising violence in Alberta's classrooms.
The federal government says its plan to increase taxes on capital gains is aimed at wealthy Canadians to achieve “tax fairness.”
At 6'8" and 350 pounds, there is nothing typical about UBC offensive lineman Giovanni Manu, who was born in Tonga and went to high school in Pitt Meadows.