WASHINGTON -- Republican lawmakers challenged the country's top medical regulator Wednesday to explain why her agency did not take action sooner against the specialty pharmacy at the centre of a deadly meningitis outbreak.
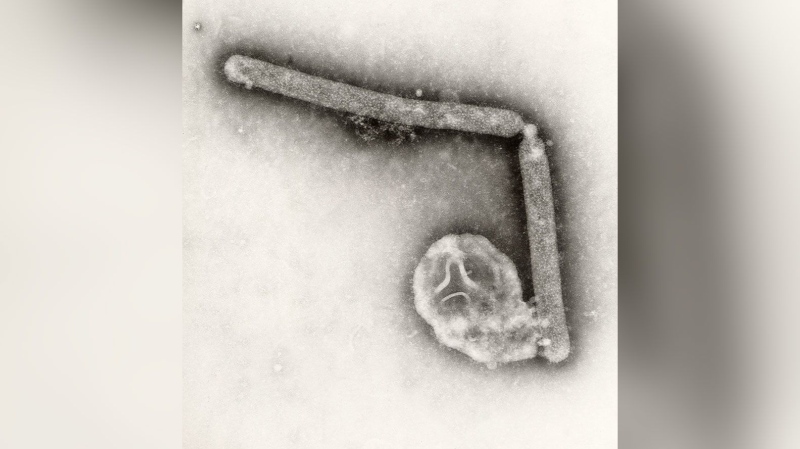
Food and Drug Administration Commissioner Margaret Hamburg testified before the House Energy and Commerce Committee, which has convened the first hearing to examine the outbreak that has sickened about 440 people and caused 32 deaths across the U.S. The illness has been tied to the New England Compounding Center, which distributed pain steroids that later tested positive for contamination.
Health officials say as many as 14,000 people received the steroid shots, mostly for back pain.
Republicans lawmakers, who make up the majority of the House, focused on NECC's history of troubles, questioning why regulators at the FDA and the Massachusetts board of pharmacy did not take action against the pharmacy years earlier.
"After a tragedy like this, the first question we all ask is: could this have been prevented?" asked Rep. Cliff Stearns, R-Fla. "After an examination of the documents provided by the Massachusetts Board of Pharmacy and the FDA -- the answer here appears to be yes.
A timeline assembled by the Republican staff shows that the FDA inspected NECC three times since 2002 for sterility issues. The Massachusetts board of pharmacy investigated at least 12 separate complaints involving the pharmacy, dating back to its founding.
"I was stunned and angered to learn that an inspection of the NECC by the FDA and the Mass Board over 10 years ago identified contamination in the very same drug at issue in the current outbreak," said Rep. Fred Upton, R-Mich., who chairs the Energy and Commerce committee.
Hamburg told lawmakers that the problems uncovered in inspections were "very serious," but that the agency was obligated to defer to Massachusetts authorities, who have more direct oversight over pharmacies.
"The challenge we have today is that there is a patchwork of legal authorities that oversee the action we can take," said Hamburg, who was nominated to head the FDA by President Obama in 2009.
In her prepared testimony, Hamburg said Congress should draft new laws and provide more funding to police large specialty pharmacies, which have long operated in an area between state and federal law.
"In light of growing evidence of threats to the public health, the administration urges Congress to strengthen standards for non-traditional compounding," Hamburg told lawmakers.
Compounding pharmacies traditionally fill special orders placed by doctors for individual patients, turning out a small number of customized formulas each week. They are typically overseen by state pharmacy boards, though the FDA occasionally steps in when major problems arise. Some pharmacies have grown into much larger businesses in the last 20 years, supplying bulk orders of medicines to hospitals that need a steady supply of drugs on hand.
Hamburg suggested putting in place a two-tier system in which traditional compounding pharmacies continue to be regulated at the state level, but larger pharmacies would be subject to FDA oversight.
Pharmacies that ship bulk product or produce complex drugs would have to register with the FDA and undergo regular inspections, similar to pharmaceutical manufacturers. These non-traditional compounding pharmacies would also have to meet the more stringent manufacturing standards required of pharmaceutical companies.
Earlier in the hearing, the owner and director of the NECC declined to testify, invoking his Fifth Amendment right to not answer questions in order to avoid self-incrimination.
After repeated questioning by House lawmakers, Barry Cadden told lawmakers: "Under advice of counsel, I respectfully decline to answer under basis of my constitutional rights and privileges, including the Fifth Amendment."
Federal officials have opened a criminal investigation of Cadden and the NECC.
The Framingham, Mass.-based pharmacy has been closed since early last month, and Massachusetts officials have taken steps to permanently revoke its license.
Inspections last month by state and federal officials found a host of potential contaminants at NECC's facility, including standing water, mold and water droplets. Compounded drugs are supposed to be prepared in temperature-controlled clean rooms to maintain sterility.
Cadden appeared immediately after the widow of a longtime Kentucky judge, who was the first confirmed victim of the outbreak.
Speaking without notes, Joyce Lovelace told lawmakers of more than 50 years of marriage to 78-year-old Eddie C. Lovelace, who was a circuit judge until he died Sept. 17 at Vanderbilt University Medical Center.
She asked lawmakers to implement laws to police companies like the New England Compounding Center, which distributed steroids that tested positive for contamination.
"My family is bitter, we are angry, we are heartbroken and devastated. I come here begging you to do something about the matter."