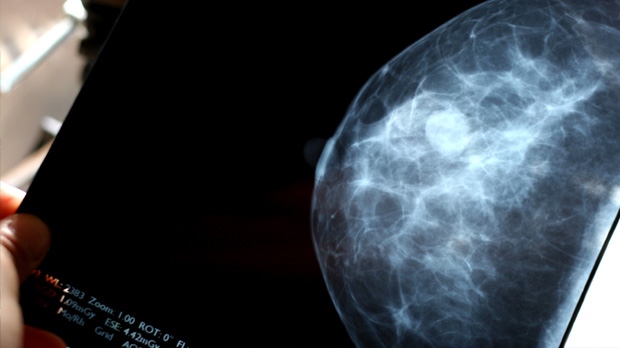
The breast cancer drug tamoxifen further reduces the risk of disease recurrence and death when the treatment period is doubled to 10 years from the current standard of five, a new study suggests.
The findings -- published this week in the journal The Lancet -- could change the standard of treatment for premenopausal breast cancer patients who take the drug.
Post-menopausal breast-cancer patients are usually prescribed different medications, such as Femara, Aromasin.
The study included more than 6,800 women with estrogen-receptor positive cancer. All had taken tamoxifen for the standard five years. For the study, half of the women were assigned to continue the drug for another five years, while the other half stopped treatment.
The researchers noticed little change in recurrence or death rates among the group that continued treatment in the five to nine years after diagnosis. However, the impact of extended treatment in the second decade after diagnosis was profound, with a 25 per cent drop in the recurrence rate and a 29 per cent decline in mortality.
“The main additional benefit from continuing tamoxifen treatment is to reduce breast cancer mortality during the second decade after diagnosis,” lead author Dr. Christina Davies said in a statement.
Davies presented the findings Wednesday at the San Antonio Breast Cancer Symposium.
Tamoxifen is a hormone blocker, and is prescribed to breast cancer patients whose tumours are estrogen-receptor positive, meaning they feed off the hormone.
The drug is proven to reduce disease recurrence and death when taken for five years. However, previous research had suggested the drug had little benefit beyond five years, so that has been the standard course of treatment for the past decade-and-a-half.
When comparing the two groups, the researchers noted that the risk of death was 12.2 per cent among the extended-treatment group and 15 per cent among the group that stopped taking tamoxifen after five years.
The greatest benefit of extending treatment was observed between 10 and 14 years after diagnosis.
While the findings would seem to be good news for premenopausal breast cancer patients, the drug’s side effects compel some women to stop treatment entirely, making extending treatment a daunting prospect.
Uncomfortable side effects include night sweats and hot flashes, but the drug can also cause more-worrisome effects, such as blood clots and endometrial cancer. Still, endometrial cancer is easy to treat, and the study found no increased risk of the disease among premenopausal women, The Lancet noted.
"Overall, the benefits of extended tamoxifen seemed to outweigh the risks substantially," Dr. Trevor Powles, of the Cancer Centre London, wrote in an editorial published with the study.
While it would seem the findings will impact the treatment regimen for premenopausal women, it remains unclear how the study will impact older breast cancer patients.
Many are prescribed Femara, Aromasin or other aromatase inhibitors, but some decide to stick with tamoxifen because it does not have some of the newer drugs’ more daunting side effects, such as joint pain, bone loss and sexual problems.
The study was sponsored by cancer research organizations in Europe, the U.S. Army, and AstraZeneca, which manufactures a generic form of tamoxifen.
With files from The Associated Press