Angela M. Christiano understands alopecia areata on more than a molecular level. The research she does on the hair-destroying disease is personal – she suffers from it herself.
So when her Columbia University Medical Center lab was able to restore the hair of three patients using an FDA-approved drug, she was able to understand the implications better than anyone else.
“It is not a life-threatening form of autoimmunity, however it is a life-altering form of autoimmune disease,” Christiano, a molecular dermatology professor, says in a video interview with the university. “It affects patients in a very profound and very personal way who undergo hair loss.”
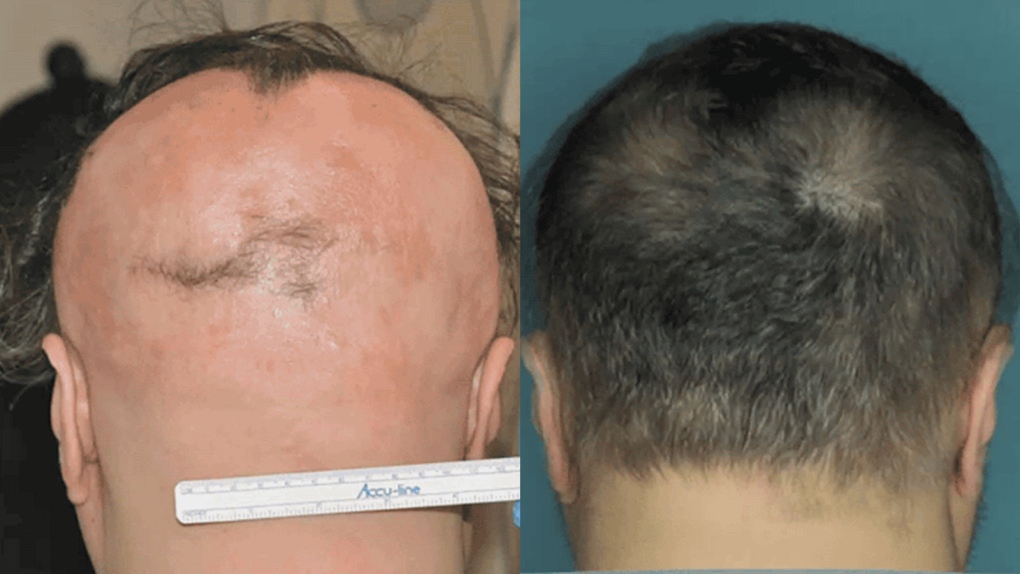
Alopecia areata can cause everything from circular bald spots in the scalp to full-body hair loss in those with the disease.
In a paper published in Sunday’s issue of Nature Medicine, Christiano and her team identified the immune cells responsible for destroying hair follicles and tested a drug called ruxolitinib on patients.
The results were dramatic -- three trial participants saw complete hair regrowth within four to five months of starting treatment, with the cells responsible for the hair loss also disappearing.
Though the study size was minimal, Christiano says interest in treating the disease is substantial.
“Over six million people in the U.S. also suffer from various forms of alopecia areata, so this is a tremendously large and motivated population in patients who have no other treatments available.”
Steady success
In previous studies, the team was able to identify a “danger signal” that prompted immune cells to attack hair follicles of patients.
First studying mice, the lab was able to identify the specific cells responsible for hair loss and identify a class of drugs -- called JAK inhibitors -- to combat the harmful cells.
After successfully regrowing hair on mice, the team put their hypothesis to the true test – human trials.
“Treatment for this disease in the past has been hit-and-miss,” says Raphael Clynes, co-lead author of the study. “We’ve been excited to see data both in mice, and now in people, to show that we can reverse this disease with small molecules that are aimed at reversing the inflammatory condition in humans.”
Committed to treatments
Ruxolitinib is currently approved by the U.S. Food and Drug Administration as a treatment for a bone marrow disorder. While doctors could prescribe it to patients with alopecia areata, it would be considered an “off-label” use – which means pharmaceutical companies wouldn’t be able to promote their drug as a treatment for the autoimmune disease.
Christiano says her team is committed to not only understanding and treating the disease, but to ensuring patients have access to the drugs that might prove to be the treatment those suffering from auto-immune hair loss have been hoping for.
“There are so many other diseases, if you will, ahead of us -- other diseases that might be considered much more like-threatening or life-altering -- that alopecia can sometimes get pushed to the backburner or to the bottom of the list,” says Christiano.
“But this has been the major focus of our entire group, and we would like very much to make sure that this ball gets across the finish line, whatever that takes.”
However, Clynes cautions that more still needs to be done before the results of her team’s research can be applied to more patients.
“We still need to do more testing to establish that ruxolitinib should be used in alopecia areata, but this is exciting news for patients and their physicians," Clynes said.