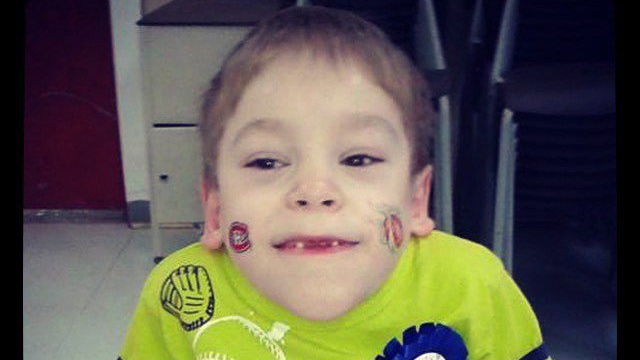
FREDERICTON -- New Brunswick's health minister says the province will provide interim coverage of a costly drug for a 10-year-old boy suffering from a rare and often fatal disease, reversing a decision the government made earlier this year.
Victor Boudreau issued a brief statement Wednesday saying the province will pay for up to a year's worth of Vimizim to assess its effectiveness in treating Morgan Doucet, who has Morquio syndrome.
The boy's mother, Carolle Mazerolle, says Morgan is in declining health because the disease causes an enzyme deficiency that leads to many complications.
As a result, the boy from Baie-Sainte-Anne, N.B., always feels sick, has headaches and often vomits. He also has a hard time breathing, uses a walker to get around and has had several surgeries.
Mazerolle said she was thrilled when she received a call from Boudreau early Wednesday confirming the province's plan to pay for a drug that costs about $200,000 annually.
"It really surprised me," she said in an interview from her home. "I don't know what changed their decision and I don't care. I'm just glad we got it ... It's a great Christmas gift."
Last week, Boudreau said he instructed health officials to find an independent specialist to offer a second opinion about the drug, made by BioMarin Pharmaceutical (Canada) Inc.
Boudreau could not be reached for comment Wednesday. However, he issued a statement saying New Brunswick will work with other provinces to devise a national approach to reviewing expensive drugs for rare diseases.
The issue is expected to be part of high-level discussions next month when provincial health ministers meet with their new federal counterpart, Jane Philpott.
Durhane Wong-Rieger, CEO of the Canadian Organization for Rare Disorders, said every province faces the same thorny questions when residents ask for help paying for pricey medications for rare ailments.
"Most rare-disease drugs don't go on to anybody's formulary," she said, referring to the list of drugs each province is willing to routinely pay for. "Quite frankly, they should not go on a formulary because they are highly specialized drugs. They need a lot of monitoring."
In the case of Vimizim, it was approved for use by Health Canada in July 2014, but before it could be added to any public drug plan it was submitted to the Canadian Agency for Drugs and Technologies in Health.
Through its Common Drug Review process, the agency recommended in March that the provinces should not add it to their formularies.
However, Wong-Rieger says the criteria for that review process was never meant to deal with rare-disease drugs.
"It was never designed to look at the ... cost-effectiveness of these highly specialized drugs," she said in an interview.
"They're not saying it doesn't work. Heath Canada has already said it works. What they're saying is, 'We don't have enough evidence that it works well enough to be worth putting so much money into it."
Still, that hasn't stopped other provinces from paying for this type of drug on a case-by-case basis.
Last month, the Saskatchewan government said it would pay for one year of Vimizim treatments for three siblings who have Morquio syndrome. Their family's funding request was initially turned down at the end of September.
Saskatchewan Health Minister Dustin Duncan has said the decision to cover the cost came after additional consultation, similar to what has happened in New Brunswick.
The drug is not a cure, though studies indicate it is effective in slowing down the disease.
Wong-Rieger says the initial decisions by the two provinces not to cover the drug was a travesty.
"We believe that every patient who has a rare condition for which there is an approved therapy ... should be given the opportunity to at least try the therapy," she said.
"This is a tragedy in our health-care system in Canada. We have no ability to assure that we have equal access across the provinces."