Doctors in Boston were able to treat diarrhea caused by C. difficile infection by giving patients capsules made up of frozen fecal material, according to a new preliminary study.
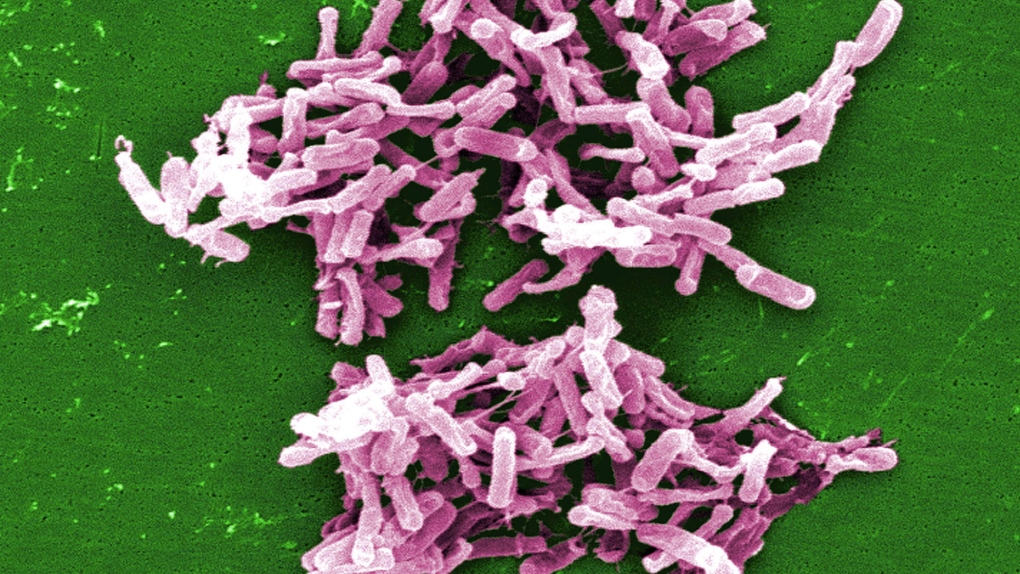
C. difficile (Clostridium difficile) is a bacterial infection that causes nausea, cramping and diarrhea. It is a major cause of illness and death across the globe, with an estimated 500,000 Americans being affected by it each year.
The infection is typically treated with antibiotics, and in some cases an experimental fecal transplant. But doctors in Boston are testing a new of treatment for C. difficile infection (CDI), that involves capsules made from the frozen fecal matter from healthy donors.
In a new study, published online Saturday in The Journal of the American Medical Association, the doctors tested the safety and effectiveness of the capsules on 20 patients with mild to moderate CDI.
The capsules were made using fecal matter from healthy volunteers, who were carefully screened.
The patients were given 15 capsules on two consecutive days, and were followed up for any resolution of their symptoms for up to six months.
Of the 20 patients, 14 had their diarrhea resolved after the first capsules were given to them. These patients remained symptom-free eight weeks after they took the capsules.
The six patients who did not respond after the first capsules were administered, were re-treated on average seven days later. Of these six patients, four had their diarrhea resolved, resulting in an overall 90-per-cent rate of diarrhea resolution.
According to the study, the daily number of patients' bowel movements decreased from a median of five the day before the capsules were administered, to two a day three days after the capsules were administered. Two months after receiving the capsules, the median daily number of bowel movements among patients was one.
Using a standardized questionnaire, the patients also reported improved health during the course of the study. The day prior to receiving the capsules, the median rating for overall health was five and the median for gastrointestinal health was 4.5. Two months after receiving the capsules, both medians increased to eight respectively.
No adverse events were observed during the course of the study.
The researchers said the study could eventually lead to making fecal matter more accessible and safer to use for a larger group of patients.
"The use of capsules obviates the need for invasive procedures for administration, further increasing the safety of (Fecal microbiota transplantation) by avoiding procedure-associated complications and significantly reducing cost," the authors say in the study. They concluded that larger studies are needed to confirm the results of their preliminary study.
Last October, Calgary's Dr. Tom Louie made headlines after he presented data at a scientific conference on a similar treatment.
At the time Louie had treated 27 patients with C. difficile with handmade pills, and all 27 of the patients saw their infections clear up.