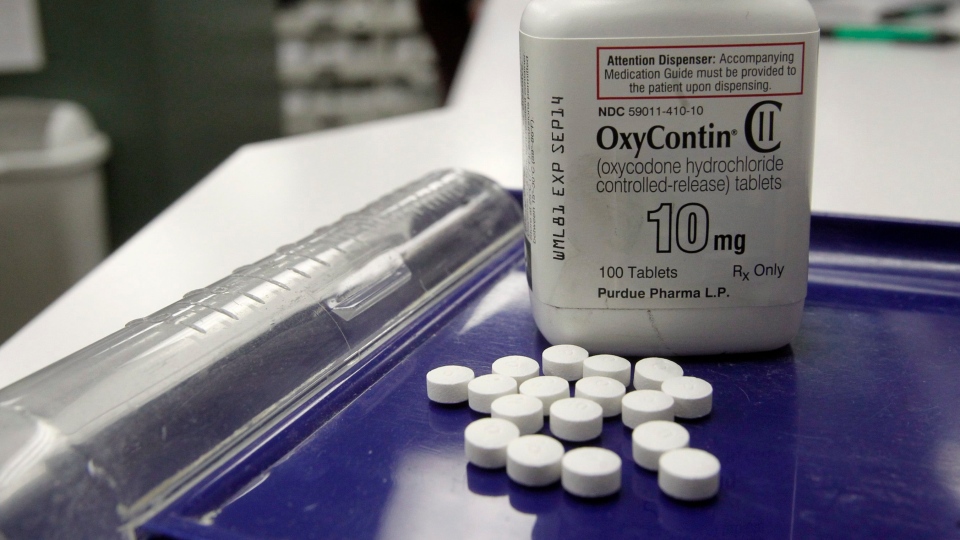
OKLAHOMA CITY -- The maker of OxyContin and the company's controlling family agreed Tuesday to pay a groundbreaking US$270 million to Oklahoma to settle allegations they helped create the nation's deadly opioid crisis with their aggressive marketing of the powerful painkiller.
It is the first settlement to come out of the recent coast-to-coast wave of nearly 2,000 lawsuits against Stamford, Connecticut-based Purdue Pharma that threaten to push the company into bankruptcy and have stained the name of the Sackler family, whose members are among the world's foremost philanthropists.
"The addiction crisis facing our state and nation is a clear and present danger, but we're doing something about it today," Oklahoma Attorney General Mike Hunter said.
Nearly US$200 million will go toward establishing the National Center for Addiction Studies and Treatment at Oklahoma State University in Tulsa, while local governments will get US$12.5 million. The Sacklers are responsible for US$75 million of the settlement.
The deal comes two months before Oklahoma's 2017 lawsuit against Purdue Pharma and other drug companies was set to become the first one in the recent barrage of litigation to go to trial. The remaining defendants are still facing trial May 28 for their role in creating what Hunter called "this nightmarish epidemic" and "the worst public health crisis in our state and nation we've ever seen."
Opioids, including heroin and prescription drugs like OxyContin, were a factor in a record 48,000 deaths across the U.S. in 2017, according to the Centers for Disease Control and Prevention. Oklahoma recorded about 400 opioid deaths that year. State officials have said that since 2009, more Oklahomans have died from opioids than in vehicle crashes.
In a statement, Purdue Pharma said the money that will go toward addiction studies and treatment in Oklahoma will help people across the country. CEO Craig Landau said the company is committed to "help drive solutions to the opioid addiction crisis."
Plaintiffs' attorney Paul Hanly, who is not involved in the Oklahoma case but is representing scores of other governments, welcomed the deal, saying: "That suggests that Purdue is serious about trying to deal with the problem. Hopefully, this is the first of many."
But some activists were furious, saying they were denied the chance to hold Purdue Pharma fully accountable in public, in front of a jury.
"This decision is a kick in the gut to our community," said Ryan Hampton, who is recovering from opioid addiction. "We deserve to have our day in court with Purdue. The parents, the families, the survivors deserve at least that. And Oklahoma stripped that from us today."
He added: "We cannot allow Purdue to cut backroom deals with state attorneys general."
Purdue Pharma introduced OxyContin in the 1990s and marketed it aggressively to doctors, making tens of billions of dollars from the drug. But the company has been hit with close to 2,000 lawsuits from state and local governments trying to hold it responsible for the scourge of addiction.
The lawsuits accuse the company of downplaying the addiction risks and pushing doctors to increase dosages even as the dangers became known. According to a court filing, Richard Sackler, then senior vice-president responsible for sales, proudly told the audience at a launch party for OxyContin in 1996 that it would create a "blizzard of prescriptions that will bury the competition."
Earlier this month, Purdue Pharma officials acknowledged that are considering bankruptcy because of the crush of lawsuits. But Oklahoma's attorney general said the company gave assurances it is not filing for bankruptcy in the near term. And he said the settlement money is "bankruptcy proof" -- that is, "it's not at risk in the event Purdue declares bankruptcy."
Purdue Pharma has settled other lawsuits over the years, and three executives pleaded guilty to criminal charges in 2007. But this is the first settlement to come out of the surge of litigation in the past few years that focuses largely on the company's more recent conduct.
Lance Lang, a 36-year-old recovering user from Oklahoma City, said he is glad some of the settlement will go toward helping those still suffering from addiction.
"My heart breaks for those that we've already lost. I've buried several myself," said Lang, who now helps recovering users find housing. "But I also know we have waiting lists of dozens and dozens for our facilities, and the state has waiting lists of hundreds and hundreds of people who need help right now."
But Cheryl Juaire, whose 23-year-old son Corey died of an overdose in 2011, said she was devastated to hear about the settlement.
Jauire, who lives in Marlborough, Massachusetts, had been organizing a group of hundreds of mothers to go to the first day of the trial and stand outside with photos of their dead children. She said a complete airing of the facts is the only way to fully hold Purdue to account.
A settlement is "a huge disservice to the tens of thousands of families here in the United States who buried a child," she said. "That's blood money from our children."
The Sacklers are major donors to cultural institutions, and the family name is emblazoned on the walls at many of the world's great museums and universities. But in the past few weeks, as the accusations have mounted, the Tate museums in London and the Guggenheim Museum in New York have cut ties with the family, and other institutions have come under pressure to turn down donations or remove the Sackler name.
A Massachusetts court filing made public earlier this year found that Sackler family members were paid at least US$4 billion from 2007 until last year.
More than 1,400 federal lawsuits against pharmaceutical companies have been consolidated in front of a single judge in Cleveland who is pushing the drug makers and distributors to reach a nationwide settlement with state and local governments.
------
Mulvihill reported from Cherry Hill, New Jersey. Associated Press reporters Claire Galofaro in Louisville, Kentucky; Sean Murphy in Oklahoma City; and Jill Bleed in Little Rock, Arkansas, contributed to this report.