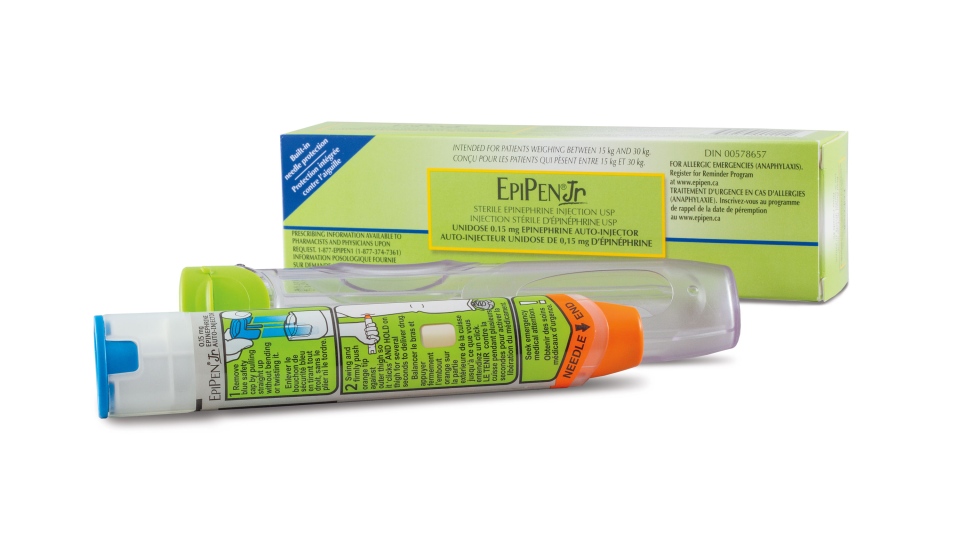
An Ontario mother says she was scared to use her son’s EpiPen when she suffered a severe allergic reaction because she didn’t know when she could replace the life-saving device.
Pfizer, the only company that makes EpiPens, announced a shortage on July 30 due to a manufacturing issue. Canada has experienced a low supply of EpiPens since at least January, but Health Canada warned that pharmacies could totally run out sometime in August as they await shipments.
So when Michelle Nel from Prescott, Ont., was stung by a hornet four times while gardening last week, she hesitated. She immediately felt dizzy, but she didn’t want to use her son’s EpiPen.
“I knew what was going on, and it wasn’t as much denial that I was having a reaction. It was that I wasn’t ready to deal with this due to the EpiPen shortage,” Nel told CTV News.
She watched her arm swell and red hives spread across her skin. Even as her throat tightened, she waited.
Nel knew that if she used the EpiPen on herself, it would leave her 10-year-old son, Nolan, who is severely allergic to nuts, soy and other foods, with just one. On several occasions, Nel says, her son has needed two doses during life-threatening allergy attacks.
“If I used one of his EpiPens -- especially living in a rural community -- I didn’t know if I could replace it. And what if he needed it?”
Nel’s condition rapidly worsened. Eventually, when she felt she might pass out, she gave herself the injection.
“To think I’m taking away his life-saving medication, even though I was having an anaphylactic reaction, it was heart-wrenching for me,” she said.
The shortage may be over soon. In a statement to CTV News, Pfizer confirmed it is shipping auto injectors across Canada, which should reach pharmacies by the end of the week.
“Additional stock is expected to be released to the Canadian market in the coming weeks,” the company said.
Dr. Susan Waserman, professor at McMaster University medical school’s division of clinical immunology and allergy, welcomes the news of supply replenishment -- but says we will have to see what additional stocks look like in the coming weeks.
“In the meantime, same advice on our end applies -- keep expired devices and use them if no current one available -- they are still effective. Check around different pharmacies, don’t fill multiple devices at one time, adults can use 2 juniors if that is all that is available,” Waserman said. “The unfortunate part for Canadians is that we are a single device country.”
Since 2017, there have been five EpiPen shortages. Nel said she can’t believe that, in 2018, a common, life-saving medication isn’t readily available in Canada.
“It’s unacceptable and it’s appalling,” she said.
Meanwhile, Health Canada is reviewing a decision by the U.S. Food and Drug Administration to approve the expiry date extension for some EpiPen products.
The FDA approved on Tuesday a four-month extension of expiration dates for specific lots of 0.3 mg EpiPens marketed by Mylan that are expired or close to expiring.
“We are doing everything we can to help mitigate shortages of these products, especially ahead of the back-to-school season,” said Janet Woodcock, director of the FDA’s Center for Drug Evaluation and Research, in a statement.
“We’re hopeful this action will ensure patients have access to this important medication and provide additional peace-of-mind to parents as the agency works with the manufacturer to increase supply.”
In a statement to CTV News, Health Canada spokesperson André Gagnon said the agency is aware of the FDA notice and is working with Pfizer to assess the information. Each country has its own regulatory process for drug approval, which includes any changes in product instructions for use.
“Health Canada will communicate a decision regarding the extension of the expiry date once we review the data that formed the basis of the FDA decision,” the statement said.
Pfizer Canada spokesperson Christina Antoniou told The Canadian Press in April that it's not unusual to have short periods of reduced supply of the auto-injector.
“There’s a relatively short shelf-life on it of 12 to 18 months," she said, “so between the relatively short shelf-life and managing the inventory supply, it's quite normal for us to experience periods of limited supply.”
With a report from CTV Medical Correspondent Avis Favaro and senior producer Elizabeth St. Philip and files from The Canadian Press