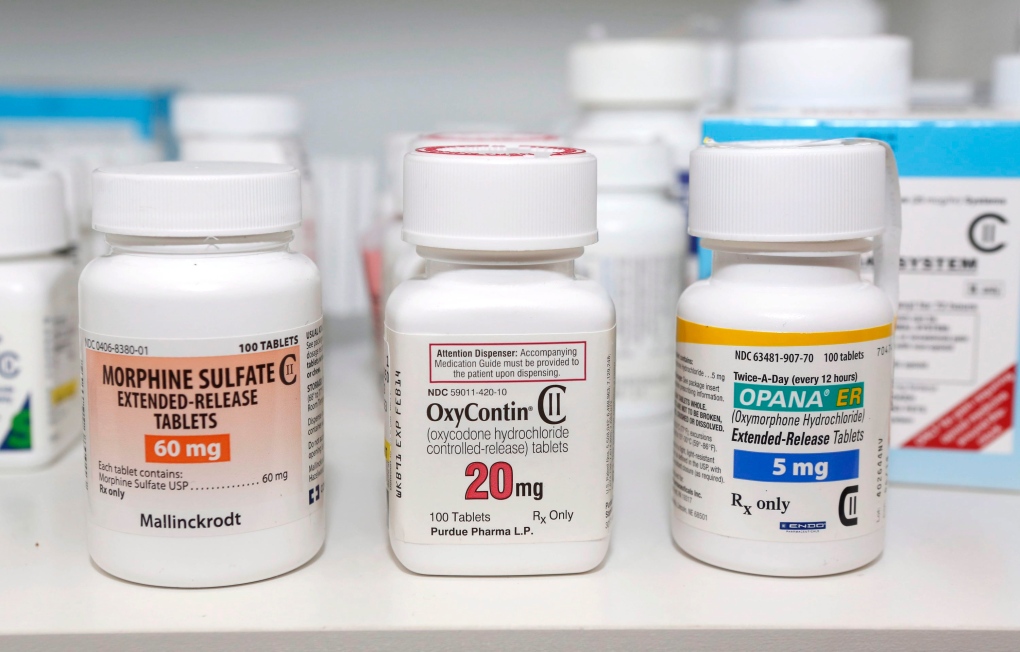
WASHINGTON -- The Food and Drug Administration has approved a new combination pain pill from the maker of OxyContin that is designed to discourage abuse by painkiller addicts.
Purdue Pharma's new drug Targiniq ER is an extended release tablet that combines oxycodone -- the active ingredient in OxyContin -- with the drug naloxone. FDA regulators approved the drug for daily, round-the-clock pain that does not respond to other medications.
If abusers crush the tablets for snorting or injecting naloxone blocks the euphoric effects of oxycodone, making the drug more difficult to abuse. Naloxone is currently used to reverse the overdose effects of opioids, highly addictive painkilling drugs including morphine, methadone, codeine and others.
The FDA notes that Targiniq can still be abused by simply swallowing the tablets, the most frequent method of painkiller abuse.
The FDA has been under intense public pressure to combat the national epidemic of prescription opioid abuse. Deaths linked to addictive medications like OxyContin and Vicodin have quadrupled since 1990 to an estimated 16,500 in 2010, the most recent year for which the Centers for Disease Control and Prevention reports figures.
Doctors prescribe opioids for a wide range of ailments, from post-surgical pain to arthritis and migraines.
Stamford, Connecticut-based Purdue has often been cited by public health advocates as a key contributor to the overprescribing of opioids. In 2007, Purdue Pharma and three of its executives paid $634 million and pleaded guilty to charges of misleading the public about the safety and addictiveness of OxyContin.
Since then the company introduced a harder-to-abuse version of OxyContin that is designed to resist crushing, chewing and dissolving.
"The FDA is committed to combatting the misuse and abuse of all opioids, and the development of opioids that are harder to abuse is needed in order to help address the public health crisis of prescription drug abuse in the U.S.," said FDA's Dr. Sharon Hertz.
The FDA is requiring Purdue to conduct long-term follow-up studies tracking rates of abuse, addiction, overdose and death with Targiniq. Those requirements are standard for all extended release opioid drugs approved in the U.S.