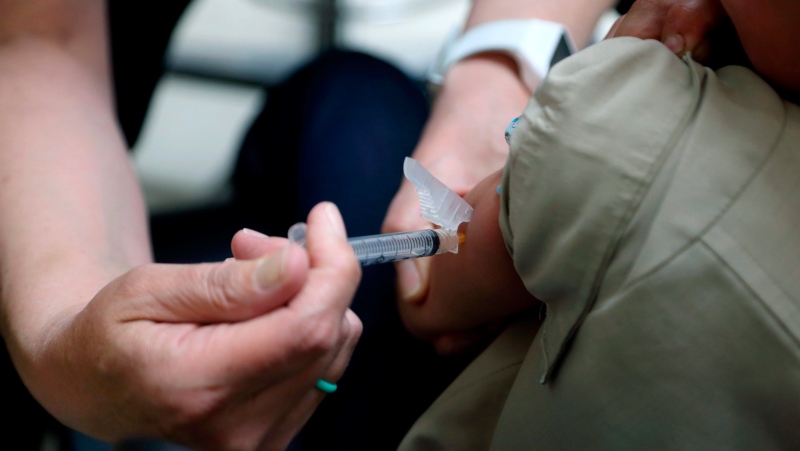
A new report says the first ever trial where oral and injectable polio vaccine were given at the same time to try to quell an outbreak has produced encouraging results.
The vaccination campaign, in and around Somali refugee camps in Kenya, resulted in high vaccination coverage, despite concerns that it would be hard to reach a lot of the children who needed the vaccine.
Follow-up surveys suggest that nearly 93 per cent of children in the camps and almost 96 per cent of children in nearby communities received the combination of oral and injectable polio vaccine, known respectively as OPV and IPV.
One hitch, however, involved the children of nomads who live in the region.
Coverage among those children was quite low, around 34 per cent; many said later they didn't know about the vaccination campaign or didn't know where to bring their children to get vaccinated.
Still, as the global campaign spearheading polio eradication efforts gets ready to start using IPV more broadly in developing countries, the Kenyan experience provides a hopeful sign it can be a useful tool.
"People, even in the public health community, have been skeptical that you actually could reach very many children in a campaign with an injectable (polio) vaccine," said Dr. Steven Wassilak, a point person with the U.S. Centers for Disease Control's polio team.
Details of the campaign and its outcome were reported Thursday in Morbidity and Mortality Weekly Report, a rapid publication journal published by the CDC.
The Global Polio Eradication Initiative is a partnership of the CDC, the service club Rotary International, the World Health Organization, UNICEF and the Bill and Melinda Gates Foundation.
Oral polio vaccine has been the workhorse of the eradication effort since its start in 1988. Cheap and easy to administer, OPV has protected billions of children from paralysis. But the vaccine has a couple of unwelcome features.
On rare occasions a child who gets the vaccine will develop polio; that happens at a rate of about one case per every 2.7 million first doses of OPV given. As well, OPV-vaccinated children shed viruses in their stools. In settings where hygiene is poor those viruses can spread to other children, immunizing them too. But if the vaccine viruses continue to circulate, they can regain the virulence that was engineered out of them in the vaccine manufacturing process. And those viruses can cause paralysis too.
(IPV, which is used in most developed countries, including in Canada, is made up of killed viruses and cannot cause polio.)
To stop those vaccine viruses from spreading, eventually the world will need to cease using OPV. Before then, in 2016, the WHO would like to take one component out the oral vaccine, which protects against three strains of polio, Types 1, 2 and 3. Type 2 viruses haven't been seen since 1999, so for several years now all Type 2 infections have been caused by the vaccine viruses.
Given that Type 2 vaccine viruses are still spreading in some parts of the world, the plan is to get all countries that use OPV to give each child at least one dose of the more expensive injectable vaccine before the Type 2 component is dropped from OPV. That would ensure all children have some protection against Type 2.
But while many experts have been pushing for more use of IPV in developing countries, there have been concerns about the feasibility of doing that.
Where oral vaccine can be and is given by trained volunteers, the injectable vaccine has to be administered by a health-care worker. There were also fears some parents might not want their children getting the unfamiliar vaccine, coming as it does in a needle. And there are concerns about the safe disposal of what could amount to millions of used syringes.
The Kenyan outbreak provided an opportunity to test the practicality of combining OPV and IPV in a developing world setting.
The outbreak, which began in April 2013, centred around refugee camps housing Somalis who had fled their war-torn country. To date there have been 217 confirmed cases of paralytic in the outbreak.
The campaign to give both the oral and injectable vaccine at one time targeted 126,000 children under age five living in the refugee camps and the surrounding communities near the Kenya-Somalia border.
The effort worked better than some skeptics might have thought, but it wasn't without problems. One child received a dose of the oral vaccine by injection and had pain and inflammation at the injection site, but recovered.
In some cases, vials of injectable vaccine -- which must be stored in a fridge -- were frozen by accident. And some problems with injection techniques were observed.
Wassilak, who was not directly involved in the study, said it showed the critical importance of adequate training of staff in a campaign like this.
Still, the results have prompted polio eradication leaders to think about trying the OPV-IPV combination out in other settings, including places like western Pakistan to which vaccination teams have a hard time gaining access.
The thought, he said, is that if teams can rarely access children in war zones or places where resistance to polio vaccine is high, using the combination might increase the chance a child will be protected. Oral vaccine alone can require multiple doses to do the trick, especially in place where children are malnourished or suffer from diarrheal diseases.
"IPV-OPV campaigns could be considered to improve population immunity and accelerate interruption of poliovirus transmission in other polio outbreaks and in certain areas where WPV (wild poliovirus) transmission is endemic," the authors of the report concluded.