Researchers in Ontario have developed a new method of transporting life-saving vaccines to remote and impoverished regions of the world at only a fraction of the cost of current techniques.
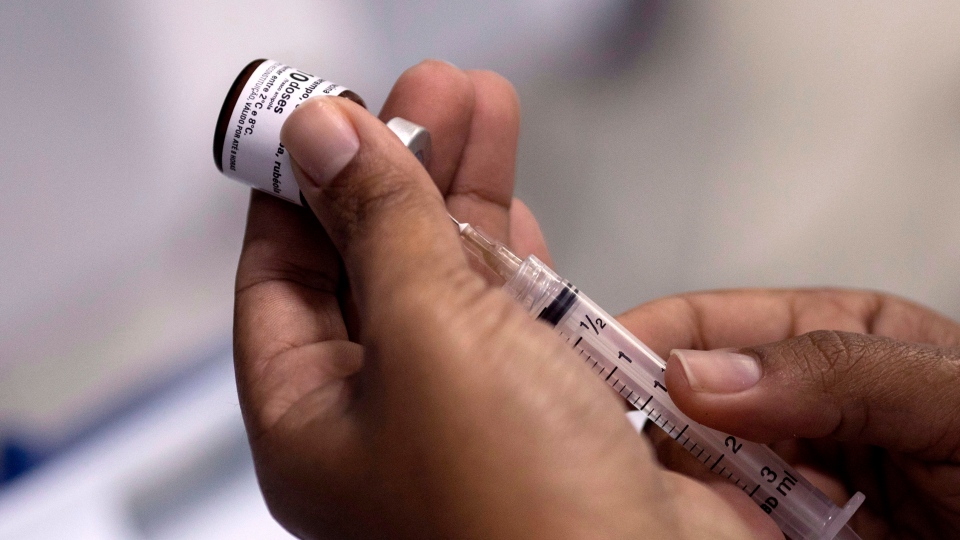
The research team from McMaster University in Hamilton, Ont. has found a way to eliminate current costly refrigeration methods or “cold-chain constant storage,” which stores vaccines at a temperature between 2 C and 8 C in order to keep them viable.
“One of the biggest problems is the need for refrigeration or what we call the cold-chain,” lead author Vincent Leung, an assistant professor of chemical engineering at McMaster University, told CTV’s Your Morning on Tuesday. “Right now, almost all vaccines on the market are required to be refrigerated from manufacturing all the way to the end user.”
The cold-chain method is considered to be a significant barrier to inoculating people in remote or poor regions of the world where they don’t always have the means or infrastructure to refrigerate and transport fragile vaccines.
“You can spend all kinds of money developing a vaccine, but if it is deactivated by high temperature an hour before you can give it to someone, it doesn’t matter,” co-author Ali Ashkar, a professor of pathology and molecular medicine specializing in immunology, said in a press release.
Leung said the new method they’ve developed could have the potential to save the lives of millions of people around the world who currently don’t have access to life-saving vaccines.
“Right now, there’s like 18.5 million children under the age of one that do not have access vaccines,” Leung said. “Every year, there’s about one and half million people that die from preventable diseases because they don’t have access to vaccines so this is a huge global health issue.”
In fact, transportation costs can often account for 80 per cent of the total cost of inoculation, according to the researchers.
In a study published Tuesday in the journal Scientific Reports, the academics share a new method of transportation that only requires two sugars that are already approved by the U.S. Food and Drug Administration (FDA) and Health Canada.
Leung said they mix the two sugars -- trehalose and pullulan – together with existing vaccines into a sugary gel. The combination allows the active ingredients in the vaccines to remain viable for eight weeks or more in elevated temperatures up to 40 C, according to the researchers.
The academics said combining the sugars with vaccines is nearly as simple as stirring sugar into coffee. They have even used the method to create an edible coating for fruits and vegetables that can prolong their shelf life.
Once the sugars are combined with the vaccine, the mixture is dried down into a thin film before transportation, Leung said. When the end user receives the vaccine, Leung said they simply have to suspend it in saline water before they can administer it to patients.
The method creates “light, durable, and compact” doses of vaccines, the researchers said, which could be ideal for shipping medicine, such as the Ebola vaccine, to affected areas in Africa. For the study, the researchers used samples of vaccines for influenza virus and herpes simplex virus to inoculate mice because they have similar immune responses to humans.
Furthermore, the research team said this way of preparing and storing vaccines eliminates nearly all of the typical costs associated with transporting inoculations.
Leung said it will still be at least three to five years before their method could be commercialized because they still need to conduct more regulatory testing to ensure it’s completely safe and easily deployable.
“To imagine that something we worked on in the lab could one day be used to save people's lives is very exciting,” he said.