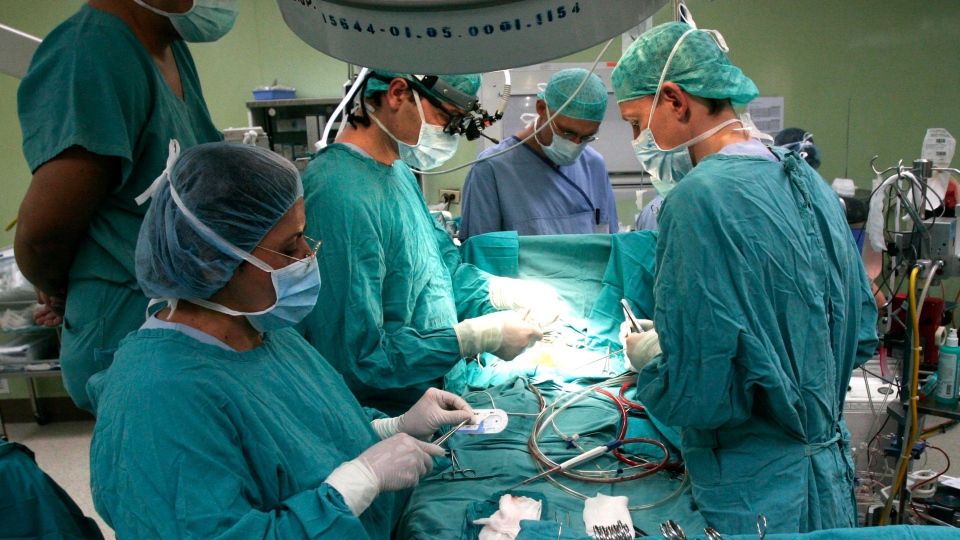
Thousands of patients in Canada who have had open-heart surgery since 2012 are being warned about a rare bacterial infection linked to medical equipment.
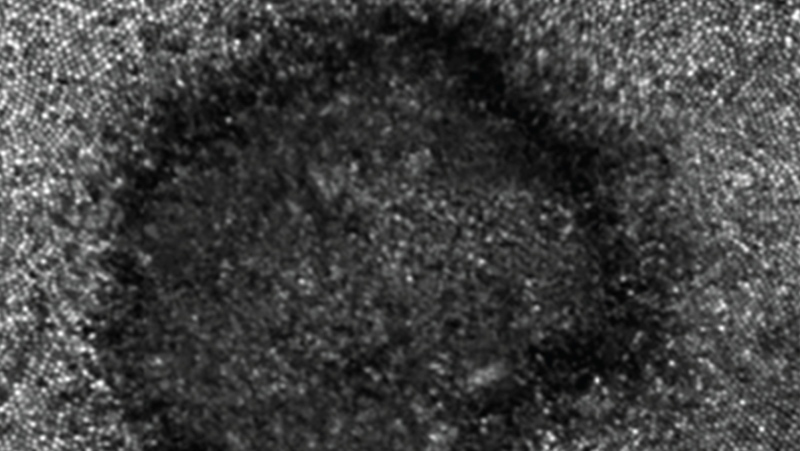
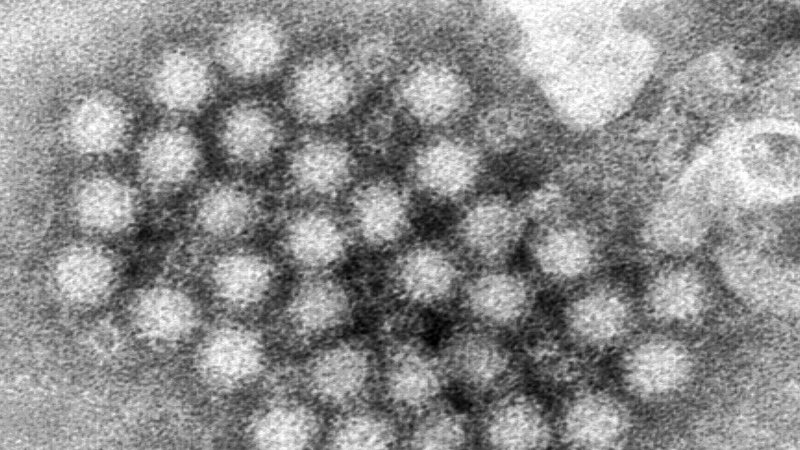
The condition is linked to non-tuberculosis mycobacteria (NTM) possibly contaminating heater-cooler devices that regulate a patient’s blood temperature during surgery. NTM is commonly found in the environment but rarely causes complications.
About 8,000 patients from Montreal Heart Institute and more than 4,300 adults who’ve had surgery at St. Boniface Hospital in Winnipeg have been notified of the potential risk.
Presently, two patients have contracted the infection in Canada and more than two dozen have been identified in the U.S.
Symptoms can take up to four years to manifest and can take more than a year of antibiotics to treat.
A recent report by CDC showed 46 per cent of those who tested positive for the bacterium died. The relatively high mortality is likely due to the lengthy amount of time it takes for symptoms to show up.
According to Health Canada’s website, symptoms include:
- Fever
- Night sweats
- Unexplained weight loss
- Muscle and joint aches
- Fatigue
- Redness, heat or pus around the incision site
There is no screening test to see if patients have been exposed during surgery. The infection is not detectable unless symptoms develop.
It was only when Dwight Blake of Delta, Pa., noticed he was getting very tired and had night sweats so bad “everything would be soaked” that he remembered a letter from his health-care provider, which warned of possible bacterial infection.
He made an appointment with his doctors to get tested, but it came back negative. It was only when he got a further blood test that doctors found the disease.
“Whether somebody tests positive or negative [...], get a full blood workup see if anything strange is going on with your kidney or liver or any other organs [...], if the blood test is showing inflammation in your system, those are much better indications [of] NTM than what growing bacteria in a petri dish will result in,” he told CTV News.
Blake is now out of the hospital but has to take a number of medications until next July.
It’s important to note that Blake’s case is very rare. The U.S. Centers for Disease Control and Prevention estimates the risk of contracting NTM is less than one per cent, and others have estimated it as low as 1 in 1,000.
However, there is still a need for increased awareness.
“If you ask most doctors they have no idea that this mycobacterium exists,” Dr. Louis Perrault, cardiothoracic surgeon at Montreal Heart Institute, told CTV News.
“The most important thing to us is to have our patients know in a preventative way that it exists and if they develop these symptoms [they] get treated as soon as possible.”
In October 2015 the CDC issued a warning to health providers on the need for increased vigilance for such infections.
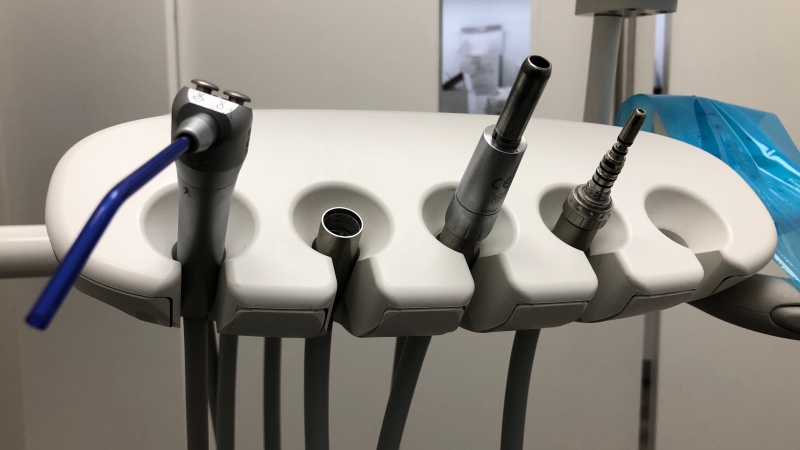
The device linked to the infection is used in hospitals across Canada, as well as the United States and Europe.
It is strongly suspected the bacteria was present in the equipment during manufacturing, but was not detected at the time.
"It is not uncommon for these devices to get contaminated," said Dr. Rakesh Arora, a cardiac surgeon with the Winnipeg Regional Health Authority.
The mycobacterium is a naturally occurring bacterium found in soil and water, including, tap water.
Usually people become infected by inhaling the bacteria, however in this instance people were exposed when condensation produced from the machine settled in their open chest.
"It is difficult to know if your machine is infected or not, so there is a deep cleaning procedure that we are using to help minimize any risk with the device," Arora said.
The heating-cooling devices are essential to performing open-heart surgeries and LivaNova, one of the manufacturers, has said they’re working with regulators to find a solution.
You can read the company’s full statement below:
LivaNova is aware of the recent CDC publication and FDA Safety Communication. We are working with regulators to develop a solution that addresses their concerns and ensures continued clinician access to this important device which enables lifesaving cardiac surgery. LivaNova and its representatives are proactively and voluntarily contacting 3T heater-cooler users to inform them of the new information in the CDC and FDA communications, and to help facilitate implementation of the agency recommendations outlined in those publications.
Heater-cooler devices are critical to regulating the temperature of patients’ blood during cardiac surgery procedures. Generally, there are no reasonable alternatives to the use of heater-cooler devices during cardiac surgery. Without these devices, hospitals would be unable to perform many of the hundreds of thousands of heart surgeries needed by patients each year.
We are working with regulators, clinicians, and all relevant parties to resolve this important industry-wide issue.
For additional information please go to our website http://www.livanova.sorin.com/products/cardiac-surgery/perfusion/hlm/3t.