Novo Nordisk's parent acquired contract drug manufacturer Catalent on Monday, as part of the Danish drugmaker's efforts to boost production of its obesity drug Wegovy when competition is heating up.
Novo Nordisk and Eli Lilly are so far the leaders in the weight-loss drug market, estimated to be worth US$100 billion by the end of the decade, with their powerful new drugs Wegovy and Zepbound, respectively.
The following is a list of publicly listed companies targeting the next big blockbuster opportunity:
Novo Nordisk
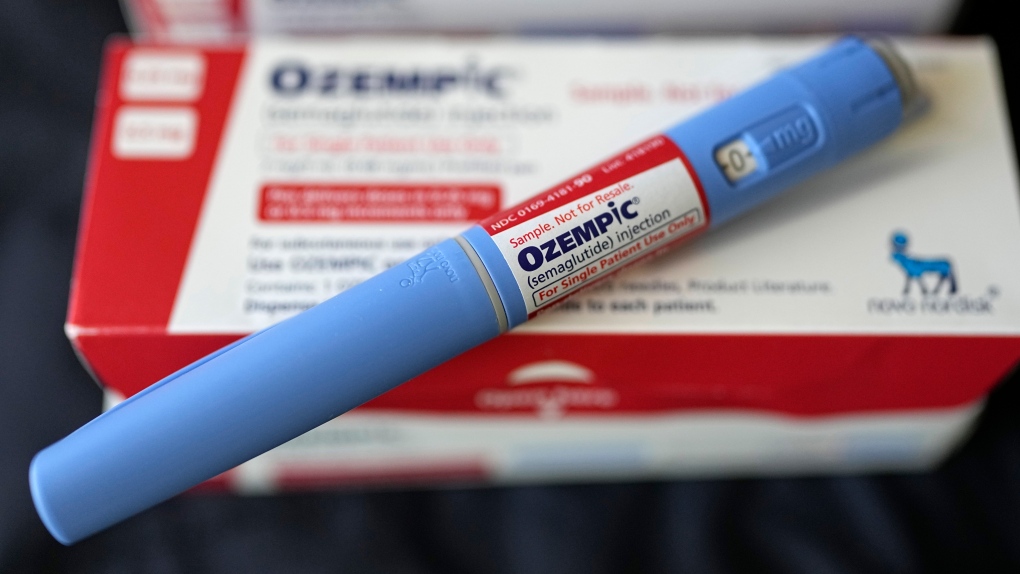
Novo Nordisk said a large study had shown its highly effective obesity drug, Wegovy, also had a clear cardiovascular benefit. Wegovy, which uses the same active ingredient as Novo's diabetes drug Ozempic, was approved in 2021.
The drugmaker in June reported late-stage trial data from a high-dose oral version of its drug, semaglutide, helping overweight or obese adults lose 15% of their body weight, which was in line with recent results for other experimental obesity pills.
The company on Monday said it will buy three of Catalent's fill-finish sites - in Anagni, Italy; Brussels, Belgium; and Bloomington, Indiana - from Novo Holdings for $11 billion to help boost its production of Wegovy. The sites will be acquired after Novo Holdings completes its Catalent acquisition.
Eli Lilly
Eli Lilly's weight-loss therapy, Zepbound, got the green light from U.S. and U.K. regulators recently, paving the way for a powerful new rival to Novo's Wegovy.
The company reported $175.8 million in sales of Zepbound in the first few weeks of its launch. The drug, chemically known as tirzepatide, has been available as Mounjaro for Type 2 diabetes since 2022 and was used "off-label" for weight loss.
Lilly said a mid-stage trial of its next-generation obesity drug candidate, a once-weekly injection of retatrutide, showed it led to a weight loss of up to 24.2% after 48 weeks.
Pfizer
Pfizer said in December it was stopping further trials of a twice-daily version of its oral weight-loss drug, danuglipron.
The decision comes after most patients in a mid-stage trial dropped out with high rates of side effects such as nausea and vomiting.
The company said it will instead focus on a once-daily, modified release version of danuglipron. Data on how this version interacts with the human body is expected next year.
Pfizer had earlier scrapped the development of its once-a-day pill in June due to concerns over liver safety.
Roche and Carmot Therapeutics
The company acquired CT-388 as part of its $2.7 billion buyout of Carmot Therapeutics. Carmot's once-a-week injection belongs to the same class as Eli Lilly's Mounjaro, or Zepbound.
The newly acquired drug candidate has completed early-stage trials and is ready to be tested on humans in the second of three trial stages, Roche said.
Amgen
Amgen's experimental obesity drug, AMG133, showed a mean weight loss of 14.5% after 12 weeks of treatment at the highest monthly dose.
Altimmune
Altimmune said its drug candidate, pemvidutide, helped reduce weight by 15.6% on average and showed continued weight loss at the end of treatment in a mid-stage trial.
However, patients also experienced nausea and vomiting of mild and moderate severity.
Viking Therapeutics
Viking Therapeutics Inc.'s drug, VK2735, showed up to 6% reduction in mean weight in an early-stage study.
The company plans to test higher doses of the drug over a longer treatment window in a mid-stage trial.
Zealand Pharma
Denmark's Zealand Pharmaand Boehringer Ingelheim's experimental obesity treatment achieved up to 14.9% weight loss in a mid-stage trial on Wednesday.
Opko Health
Opko Health has completed a mid-stage trial of its obesity drug, pegapamodutide, which it expects will have fewer side effects.
(Reporting by Mariam Sunny, Pratik Jain, Sriparna Roy and Leroy Leo in Bengaluru; Editing by Sriraj Kalluvila)