A class-action lawsuit has been filed in Canada by women who say their babies were harmed by a powerful anti-nausea drug not technically approved for use in pregnant women.
Like most mothers, Terra Mercer wanted a drug-free pregnancy. But early in her pregnancy with her daughter, Aaleyah, Terra suffered severe nausea and vomiting.
Still in her first trimester, she tried to handle it on her own, but when she began seeing blood in her vomit, she went to the hospital. A physician offered her a medication delivered by IV.
"Before I said yes, I first asked if there were any side effects. He said. ‘No, it’s safe.’ So I trusted him,” she recalled.
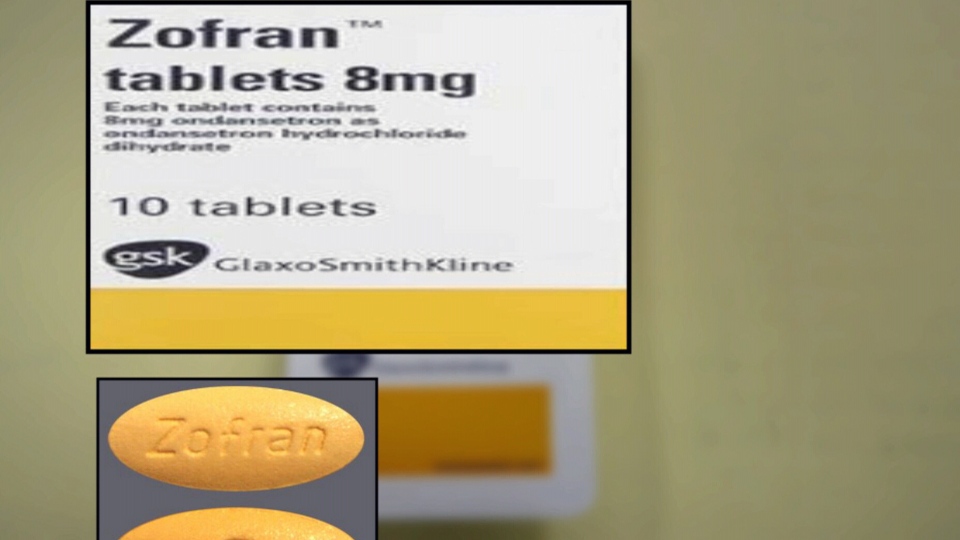
The drug was called ondansetron. It is marketed under the brand name Zofran. It is available in IV and pill form and is approved by Health Canada to prevent nausea and vomiting in chemotherapy and surgery patients.
But it is also prescribed “off label,” to treat extreme cases of morning sickness, or Hyperemesis Gravidarum , even though its possible risks have not been fully studied.
When Aaleyah was born a few months later, she emerged with a cleft lip and cleft palate. She is now facing a future of several surgeries to treat the birth defect as she grows.
Mercer believes the drug is at fault. There have not been many studies on ondansetron’s effect in pregnancy, as the drug has not been approved for that use. Still, some small independent studies have noted a link:
- Medications Used to Treat Nausea and Vomiting of Pregnancy and the Risk of Selected Birth Defects
- Ondansetron Use In Early Pregnancy And The Risk Of Congenital Malformations
Mercer says she would not have taken it if she had known that some studies have found an association between Zofran use in the first trimester of pregnancy and birth defects.
She is now one of a dozen Canadian women who have filed a class action lawsuit against two companies that make the drug, saying the companies failed to warn doctors.
“The real heart of this case is the warning about this information should be made available to health care providers and in turn, to the mothers carrying these children,” says lawyer Jill McCartney with Siskinds LLP.
The product monograph by one of ondansetron’s manufacturers, GlaxoSmith, reads:
“The safety of ondansetron for use in human pregnancy has not been established. Ondansetron is not teratogenic in animals. However, as animal studies are not always predictive of human response, the use of ondansetron in pregnancy is not recommended”
The FDA issued a warning about ondansetron, cautioning against its use in pregnancy.
Still, some doctors prescribe it to very ill pregnant women because it works to relieve severe nausea and because some studies have found no link with any birth defects.
Canada’s Society of Obstetricians and Gynecologists says lifestyle and dietary changes should be the first line of approach with morning sickness. A drug called diclectin, which is a doxylamine and pyridoxine (vitamin B6) combination, can also help, as well as another medication known as metoclopramide or Maxolon.
Zofran should only be used as a last resort, say guidelines.
Recommended treatments:
Metoclopramide (Maxeran) is another medication that is used for nausea and vomiting during pregnancy and its safety has also been studied:
Scientists such as Dr. Nav Persaud say there needs to be more studies to clarify the risks and benefits of all morning sickness drugs.
“All women should be presented with a variety of options including non-medical treatments for nausea and vomiting and also the several different medications that could be taken," he says.
As for little Aaleyah, she is healing well from her most recent surgery to repair her lip and palate while her mother is hoping other pregnant women hear the full information about morning sickness drugs that she didn't get.
With a report from CTV medical specialist Avis Favaro and producer Elizabeth St. Philip