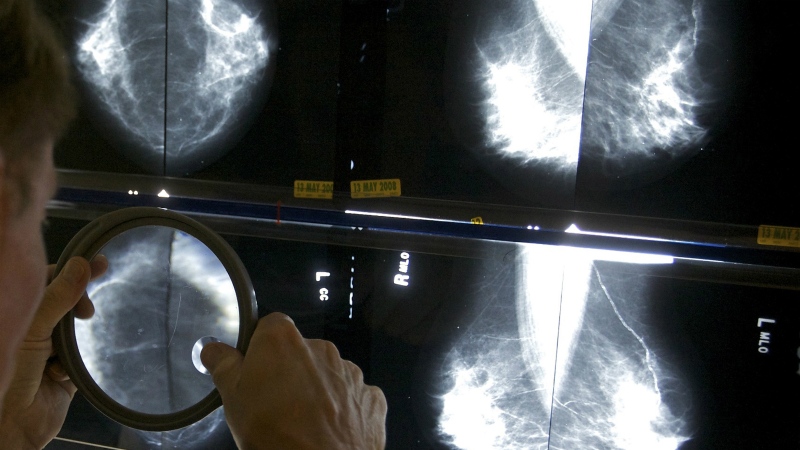
Following a safety review, Health Canada has suspended the sale and use of Allergan’s Biocell textured breast implants due to a “rare but serious risk” of cancer.
After a 2017 safety review on “breast implant-associated anaplastic large cell lymphoma” (BIA-ALCL), licences for Allergan’s Biocell textured breast implants have been suspended, Health Canada said in a release today.
Health Canada found the rate of BIA-ALCL is “significantly higher” in patients with textured breast implants compared to other types, and said the “potential risks outweigh their [sic] benefits.”
Allergan has the only macro- textured breast implants available in Canada.
The suspension means no one can sell the textured implants or import them – and Allergan has voluntarily agreed to recall unused textured breast implant products from the Canadian market, Health Canada said.
Allergan was given two weeks to provide evidence that the benefits of their product outweighed the risk, after a Health Canada announcement of intended suspension earlier this year in April.
Health Canada said they received a reply from Allergan on April 17, but expert review determined there was insufficient evidence to allow Allergen to continue to sell the product in the Canadian market.
Twenty-six confirmed Canadian cases of BIA-ALCL were brought to Health Canada’s attention, and 22 of the cases (85 per cent) involved Allergan’s Biocell textured breast implants.
BIA-ALCL is a rare type of cancer that affects the immune system, and Health Canada says it may “develop many months or years after the breast implant procedure.”
Health Canada said anyone who has Allergan Biocell textured implants (or any other implants), but no signs or symptoms of BIA-ALCL, that implant removal is not recommended and to speak to their healthcare provider about concerns they may have.