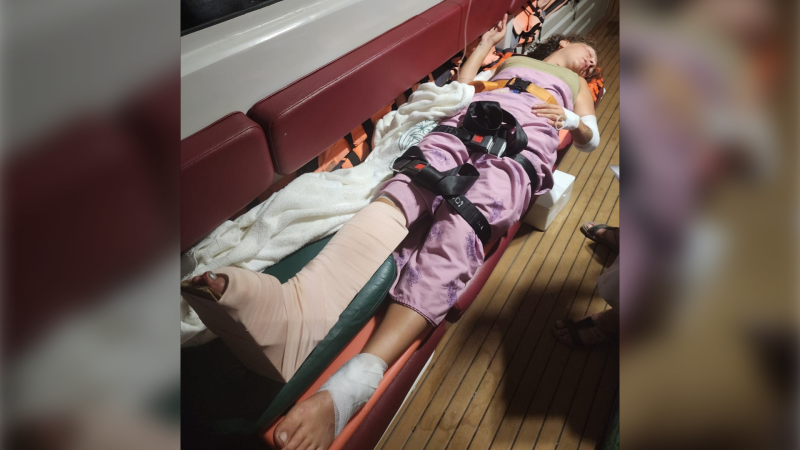
B.C. woman facing steep medical bills, uncertain future after Thailand crash
The family of a Victoria, B.C., woman who was seriously injured in an accident in Thailand is pleading for help as medical bills pile up.
The U.S. Food and Drug Administration has raised concerns about a possible risk of heart inflammation from Novavax Inc.'s COVID-19 vaccine, even as the company's data showed it could reduce the chances of mild-to-severe disease.
In Novavax's nearly 30,000 patient trial, conducted between December 2020 and September 2021, there were four cases of a type of heart inflammation called myocarditis detected within 20 days of taking the protein-based shot.
"These events raise the concern for a causal association with this vaccine, similar to the association documented with mRNA COVID-19 vaccines," FDA staff wrote in briefing documents released on Friday.
Shares of the company fell nearly 14 per cent after the FDA's analysis of data from the company's trial.
The agency said it had requested Novavax to flag myocarditis and another kind of heart inflammation called pericarditis as an "important identified risk" in its materials. The company has not yet agreed to do so.
Novavax, in response to the safety concerns flagged by the FDA, said natural background events of myocarditis can be expected in any sufficiently large database.
"Based on our interpretation of all the clinical data supporting NVX-CoV2373 ... we believe there is insufficient evidence to establish a causal relationship," the company said in a statement.
One patient in the trial reported myocarditis after receiving placebo.
Novavax has said the shot, NVX-CoV2373, will play a role in driving vaccination among those who have been hesitant to get immunized and it has started an educational effort on vaccine choices.
"Despite the wide availability of authorized or approved vaccines, the SARS-CoV-2 pandemic is not well controlled in the U.S. ... there remains a desire for vaccines that have been developed using well-understood technology platforms," it said.
The FDA analyzed data from Novavax's trial before the Omicron and Delta variant became the dominant strains.
"Based on the efficacy estimate in the clinical trial of this vaccine, it is more likely than not that the vaccine will provide some meaningful level of protection against COVID-19 due to Omicron, in particular against more severe disease," the FDA staff said.
The vaccine showed an efficacy of 90.4 per cent in Novavax's study, which enrolled adults across the United States and Mexico.
The FDA's comments came in a briefing note initially prepared ahead of a May 7 meeting of the agency's outside advisers.
Its staff comments will be used by those advisers to guide their decision on whether or not to recommend authorizing the vaccine on Tuesday. The FDA is not mandated to follow the advise of its outside experts, but usually does.

The family of a Victoria, B.C., woman who was seriously injured in an accident in Thailand is pleading for help as medical bills pile up.
Canadians will learn Tuesday the entirety of the federal Liberal government's new spending plans, and how they intend to pay for them, when Deputy Prime Minister and Finance Minister Chrystia Freeland tables the 2024 federal budget.
An Ontario woman was shocked to find she’d been charged nearly $7,000 after unknowingly using an unauthorized taxi company while on vacation in January.
As Canadians deal with a crushing housing shortage, high rental prices and inflationary price pressures, now Equifax Canada is warning that Canadian consumers are increasingly under stress"from the surging cost of living.
An inmate who escaped from Dorchester Penitentiary in Dorchester, N.B., on Saturday evening has a long history of violent crimes and a history of escaping custody.
The annual inflation rate ticked higher in March compared with February, boosted by higher prices for gasoline, Statistics Canada said Tuesday.
Tim Hortons is launching flatbread pizzas nationally in a bid to pick up more afternoon and evening customers.
Ontario Provincial Police are investigating the theft of "several thousand" dollars worth of tropical fish stolen from an Upper Ottawa Valley restaurant last week.
NASA confirmed Monday that a mystery object that crashed through the roof of a Florida home last month was a chunk of space junk from equipment discarded at the International Space Station.

Just steps from Parliament Hill is a barber shop that for the last 100 years has catered to everyone from prime ministers to tourists.
A high score on a Foo Fighters pinball machine has Edmonton player Dave Formenti on a high.
A compound used to treat sour gas that's been linked to fertility issues in cattle has been found throughout groundwater in the Prairies, according to a new study.
While many people choose to keep their medical appointments private, four longtime friends decided to undergo vasectomies as a group in B.C.'s Lower Mainland.
A popular highway in Alberta's Banff National Park now has a 'no stopping zone' to help protect two bears.
B.C. resident Robert Conrad spent thousands of hours on Crown land developing an unusual bond with deer.
A Sudbury woman said her husband was bringing the recycling out to the curb Wednesday night when he had to make a 'mad dash' inside after seeing a bear.
A school teacher who took part in the Quebec version of the Survivor reality TV show took time off work to be a contestant is now out of a job.
A young actor from Prince Edward Island is getting the chance to fulfill a childhood dream, playing the precocious and iconic Anne Shirley on stage.