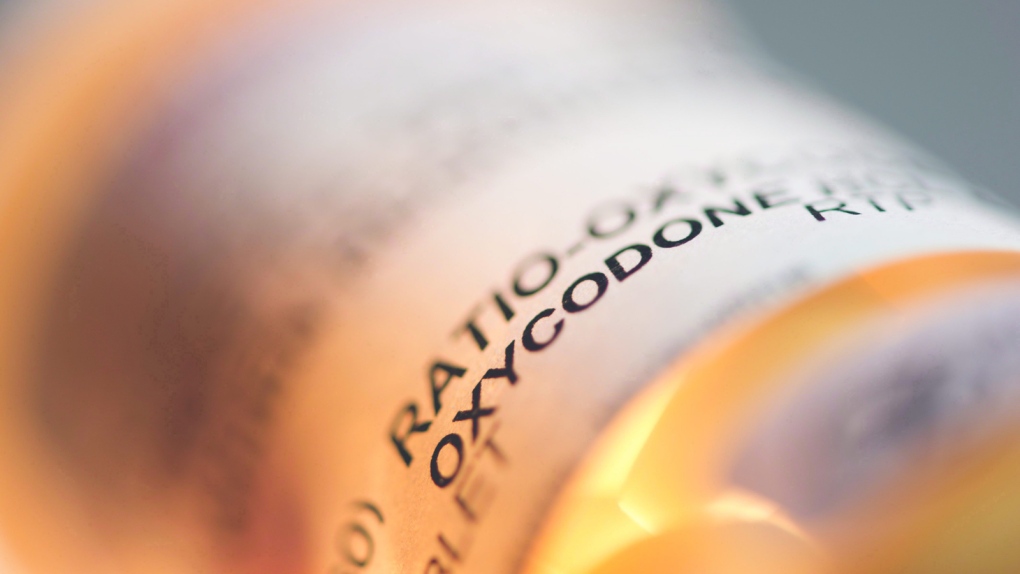
TORONTO -- Health Canada has changed the labelling for controlled release opioids in a bid to make clear the risks and safety concerns of the pain medications.
The department says the new labelling spells out more clearly the potential problems related to the drugs and to encourage more appropriate prescription of the medication.
The new labelling drops reference to use of the drugs for moderate pain, stressing that they are meant to be prescribed for pain severe enough to require daily and continuous opioid treatment.
An Ontario study released earlier this summer found that fatal overdoses from drugs such as oxycodone and morphine have soared over the past two decades as use of the addictive and highly potent painkillers has expanded.
Health Canada says that while the drugs provide effective pain management, they can induce serious health risks including accidental overdose and death, even if taken at recommended doses.
It says the label changes are an attempt to reduce these risks, which include addiction, misuse and abuse.
Health Canada says there are currently 38 brand-name and generic controlled-release opioid medicines licensed for use in Canada. Similar labelling changes will be implemented soon for generic opioids.