Senators in Canada are deliberating new regulations that would mandate closer monitoring of side effects of "natural health products."
A part of Bill C-47, which implements several commitments made by the federal government under Budget 2023, would incorporate natural health products, such as herbal remedies and supplements, into Vanessa's Law, which requires hospitals to report any adverse reactions associated with the products.
Here's what you need to know about natural health products, the risks associated with these products, and Canada’s proposed regulations.
WHAT ARE NATURAL HEALTH PRODUCTS?
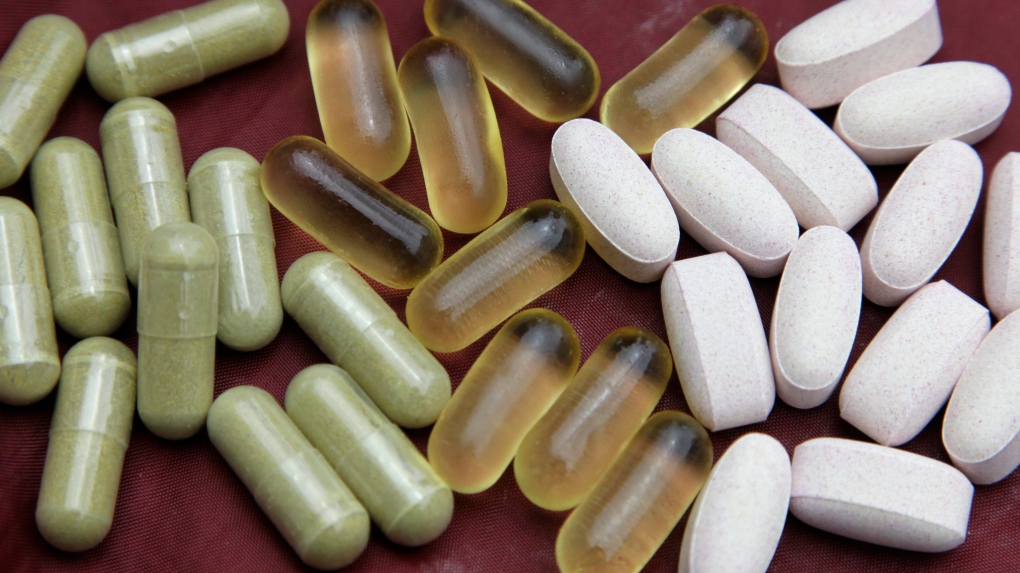
Health Canada defines natural health products as "naturally occurring substances that are used to restore or maintain good health." Also called "alternative" or "complementary" medicines, they can come in the form of tablets, capsules, tinctures, solutions, creams, ointments and drops.
Examples of natural health products include vitamins and minerals, herbal remedies, homeopathic and traditional medicines, and probiotics.
Health Canada says some everyday consumer products, such as toothpastes and shampoos, can also be defined as a natural health products in Canada, as long as the product is involved in:
- the diagnosis, treatment, mitigation or prevention of a disease, disorder or abnormal physical state or its symptoms in humans;
- restoring or correcting organic functions in humans; or
- modifying organic functions in humans, such as modifying those functions in a manner that maintains or promotes health.
WHAT ARE THE RISKS OF USING NATURAL HEALTH PRODUCTS?
Health Canada says these products are "generally safe and have fewer side effects than medications," but notes that the products are "not risk free."
According to the health agency, risks include manufacturing problems, unproven claims, a lack of information for consumers, the interaction with other drugs or natural health products and possible unwanted side effects.
The National Center for Complementary and Integrative Health—the U.S. government agency that regulates these products south of the border—also notes just because an ingredient has "natural" origins and wasn't synthetically manufactured doesn't mean it's completely safe.
For example, the agency says kava, a plant native to the South Pacific that has been used as a dietary supplement, may be associated with severe liver damage.
Some critics argue Health Canada hasn't been doing an adequate job of keeping potentially unsafe products away from consumers. A 2021 report from the Office of the Auditor General found 88 per cent of the natural health products reviewed were advertised with misleading product information. Some of the products had unproven and unauthorized health claims, wrong dosages, incomplete lists of ingredients, or unreadable information on the product label.
HOW WILL CANADA'S PROPOSED REGULATIONS AFFECT NATURAL HEALTH PRODUCTS?
Since 2014, hospitals have been required to report adverse health reactions associated with any pharmaceuticals after the federal government passed Vanessa's Law.
At the time, natural health products were excluded from these reporting requirements. But these new regulations proposed under Bill C-47 would bring natural health products into the same framework as Vanessa's Law.
The Canadian Health Food Association, which represents the natural health products industry, says it feels blindsided by the proposed regulations, arguing that the proposals haven't been properly studied or debated, instead being tucked into an omnibus budget bill.
Meanwhile, the Canadian Pharmacists Association has come out in support of the regulations, and said natural health products should've been included when Vanessa's Law was originally passed back in 2014.
With files from The Canadian Press