OTTAWA -- Health Canada has approved the Pfizer-BioNTech COVID-19 vaccine for use in this country, and the first doses could be administered as early as next week.
It’s a critical moment in Canada’s fight against the novel coronavirus, as it is the first vaccine to receive the green light.
The federal health agency has deemed the vaccine effective and safe for use on Canadians, which means that the team responsible for the rollout of vaccines can now begin the process of administering them.
“We expect vaccines to arrive maybe as early as Monday. It takes time to prepare the vaccine,” said Maj.-Gen. Dany Fortin, the top military general leading the rollout from the Public Health Agency of Canada, saying the first shots could be given by the middle of next week.
The approval comes alongside an update to the timeline for national mass vaccination effort. The plan is now to begin vaccinating the general population in April 2021, and have all Canadians immunized by the end of next year, with one of the several vaccines under consideration.
“This is a big deal,” said Prime Minister Justin Trudeau in the House of Commons Wednesday afternoon, thanking doctors, researchers and scientists who worked to approve the first COVID-19 vaccine. “We will see 30,000 vaccines begin to arrive next week, with many more on the horizon. But we are not through this yet, we've got a tough winter to get through and I know we're going to be able to get through it together.”
“Health Canada has determined that the Pfizer-BioNTech vaccine meets the Department's stringent safety, efficacy and quality requirements for use in Canada,” said Health Canada in a statement, alongside a series of documents related to the decision, with the promise of more information about the clinical trial in the weeks ahead.
In a press conference, Health Canada’s chief medical adviser Dr. Supriya Sharma called the approval a “critical milestone in our fight against COVID-19 and in our efforts to provide every Canadian with access to a vaccine,” and said extensive work from a range of scientific experts went into issuing this approval.
“We concluded that there was strong evidence supporting that the benefits of the vaccine outweigh the risks,” Sharma said. “We know that even the best vaccines will only be effective if people trust them, and ultimately agree to receive them. An important part of building trust is openness and transparency, ensuring that people have as much information as possible, to help them make informed decisions for themselves and for those they care for,” she said.
Pfizer trials concluded that the vaccine was effective at preventing COVID-19 in 95 per cent of patients, one week after the second dose is given. In terms of the long-range immunity the vaccine may provide, that is still under evaluation.
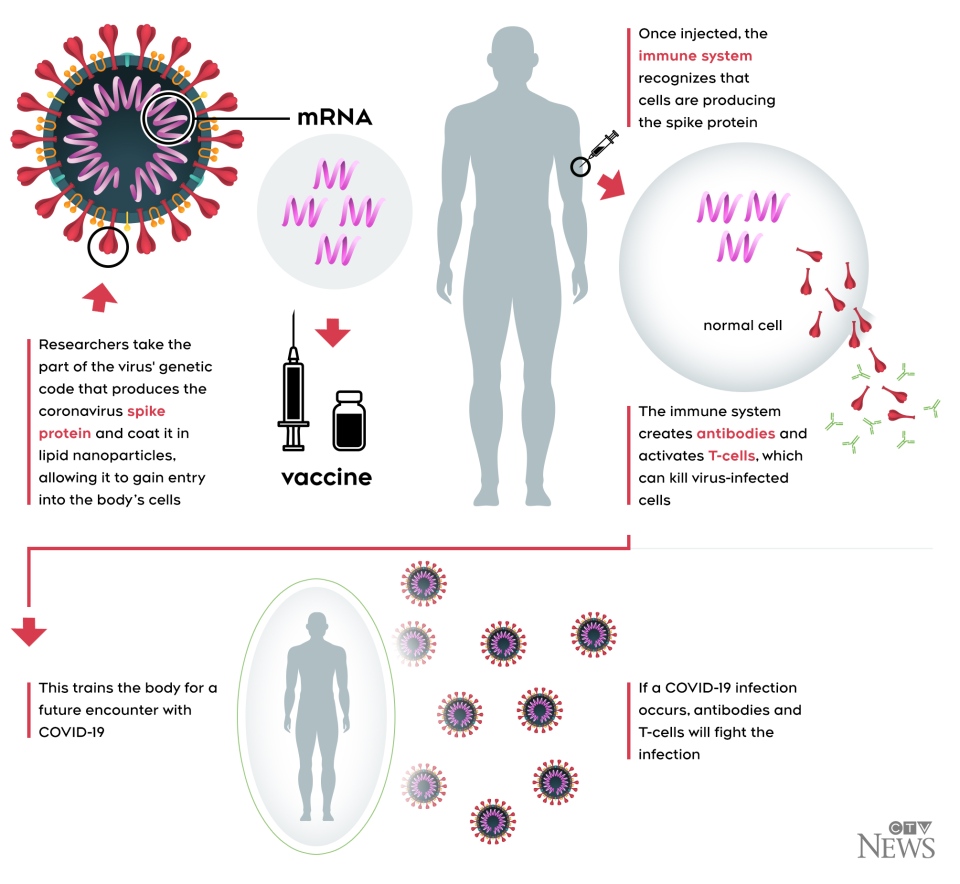
The vaccine is an mRNA vaccine, which means it teaches cells how to make a protein that triggers an immune response, without using the live virus that causes COVID-19. Once that immune response is triggered, antibodies are produced, which protect people from being infected should the virus enter their system in the future.
In an interview with CTV National News Medical Correspondent Avis Favaro, Pfizer Canada’s Vaccines Medical Lead Dr. Jelena Vojicic said she is “very pleased” with Health Canada’s decision.
“This is certainly a historic moment for science, and for Canadians. And this is a result of a tremendous effort, starting with the international scientific community, and then going over the dedicated work of Pfizer and BioNTech employees, the clinical trial sites, the participants in the clinical trials, the volunteers,” Vojicic said.
“And of course, I need to acknowledge the tremendous work by Health Canada in quickly reviewing our file while maintaining really gold standards of review and keeping a vigilant eye on the data.”
The initial doses of the Pfizer vaccine are expected to arrive in Canada next week, and plans are already in place to have the shots ready to be administered at 14 delivery sites in major cities across Canada, within one or two days of shipments arriving.
SHIPPING DOSES 'IMMINENTLY'
Vojicic said that Pfizer is prepared to ship to Canada and she is expecting that shipment will happen “imminently.” She anticipates most vaccines destined for Canada will be coming out of Belgium.
Fortin said he expects doses to be shipped by the end of the week, likely Friday.
By the end of December, Canada is set to receive up to 249,000 doses of this vaccine, or enough to vaccinate 124,500 people, given it requires two 0.3 ml shots into the muscle of the arm, 21 days apart. In total, the federal government has purchased 20 million doses of the vaccine, and has option to buy 56 million more.
From there, Fortin is expecting a “constant flow” of doses to arrive -- up to four million Pfizer doses and possibly two million of the Moderna vaccine candidate which is next in line for approval, by the end of March 2021. Moderna’s candidate is now the most advanced in Canada's regulatory process, but there isn’t a date or estimate yet for when it may be approved. That vaccine also requires cold storage but not as cold as Pfizer, meaning the logistical rollout of Moderna doses is expected to be less challenging.
That means that by the end of March, Canada is planning to have three million Canadians—or eight per cent of the population—immunized. From April and June, between 15 and 19 million Canadians will be immunized, which equates to between 40 and 50 per cent of the population. Then, between September and December the plan is to see all 38 million Canadians vaccinated. These latest projections are based on anticipated delivery schedules and are dependent on regulatory approvals of additional vaccines.
Prioritized groups will be the first to receive the vaccine, given the limited quantities to begin with. Among the earliest to receive these shots will be staff and residents in long-term care and other congregate senior living facilities and health-care workers with high exposure risks. Each province is able to modify the national recommendations for prioritization based on their regional situation. For example, Ontario has opted to use the first small batch in Toronto and Peel region, where the most severe lockdowns are in place due to weeks of surging case counts.
Reacting to Wednesday’s news, Ontario Premier Doug Ford said that “light at the end of the tunnel grows brighter."
For now, the vaccine is being recommended for use in people 16 years of age or older, and further clinical trials are being run on children of all age groups, so it’s possible the Health Canada approval could be revised in the future to include children, if the data from these studies support it.
Because the vaccine needs to be stored at temperatures below -70 C, Pfizer will be delivering batches in special thermal shipping boxes it developed that can keep the vaccine stable for days.
“These thermal shippers are also equipped with GPS-enabled data loggers that records the temperature as well as the location of these shippers. So at any point of time, between the manufacturing site and the point of use, we will be able to track those shipments and prevent any unwanted temperature excursions,” said Vojicic, who added she doesn’t think the security of theses shipments will be an issue.
Before being injected, the vaccine is thawed, decanted, and mixed, but can only last a few hours at room temperature, so Pfizer is requesting the first doses be given on-site at these 14 medical facilities where there are ultra-cold freezers in place, to avoid as much wastage as possible from transporting the vials elsewhere. This means Canadians who live in the territories and other remote areas will likely be waiting until the Moderna vaccine is approved to be able to receive a COVID-19 vaccine.
CANADA THIRD IN WORLD
Canada is the third country in the world to approve the Pfizer-BioNTech vaccine.
Bahrain approved it first, followed by the United Kingdom, which began vaccinating its citizens with this vaccine on Tuesday, though their Medical and Healthcare Products Regulatory Agency is now warning that people who have a history of serious allergic reactions should not receive the vaccine as they investigate two instances of adverse reactions that occurred in health workers when they received the vaccine.
In general the side-effects reported during the clinical trials are similar to those of other vaccines, and are considered “mild or moderate.” They include pain at the site of injection, body chills, feeling tired and feeling feverish.
The United States Food and Drug Administration is set to give the Pfizer-BioNTech vaccine the green light to roll out to Americans this week.
For weeks, questions had been raised about Canada’s place in line for vaccines in comparison to other nations, given in part our inability to domestically produce initial vaccines. But Pfizer said Wednesday that “you can see from what's happening today, that we're definitely not at the back of the line. We're actually at the front of the line in terms of the approvals of the vaccines, and the rollout of the vaccines.”
“I think we can be very content as to how Canada has done,” Vojicic said.
Asked how Canada was able to beat the U.S. FDA in deeming the vaccine safe, Sharma said jokingly “we’re just better.”
“We're not in a race with any other regulator, we're not trying to beat any other regulator, what we're trying to do is beat this… virus and working against this virus,” she said.
“It just so happened we got the last piece of information late last night, people worked at it, they were up early this morning to get it ready… that's why we're announcing today,” Sharma said, adding that Canadian officials will be participating in the U.S. meeting happening over the next few days to discuss their approval of Pfizer.
Typically, the vaccine submission review process can usually take up to a year, but because of an emergency order, Health Canada has been able to expedite the authorization process. The agency began its regulatory review of the Pfizer vaccine in October, and has since been assessing rolling information as it comes in from the pharmaceutical company’s studies, rather than having to wait until the end of its work to begin reviewing the findings.
“Canadians can feel confident that the review process was rigorous and that we have strong monitoring systems in place. Health Canada and the Public Health Agency of Canada will closely monitor the safety of the vaccine once it is on the market and will not hesitate to take action if any safety concerns are identified,” said Health Canada on Tuesday.
The pharmaceutical giant will also have to routinely provide additional quality, efficacy, and safety information. Pfizer has agreed to follow clinical trial participants for two years after their second dose, and will be releasing before March 31 a full risk management plan that covers the known and potential safety issues, plans for collection of additional safety and effectiveness information, and measures that will be put in place to minimize risks associated with the product.
Plans are being made for each province and territory to track who receives doses, and the federal government is considering an additional layer of national monitoring, both for overall immunization levels, and adverse reactions.
Pfizer was one of four vaccine candidates Health Canada has been evaluating, with assessment ongoing for the Moderna, AstraZeneca, and Johnson & Johnson vaccines. In total, Canada has signed contracts guaranteeing access to 194 million doses of potential COVID-19 vaccines with the option to purchase 220 million more, meaning if all trials pan out, we’d have access to 414 million doses.
Six of the seven vaccines require two doses, with the Johnson & Johnson vaccine candidate being the exception. For those that require two doses, all but the Moderna and Astra Zeneca require their doses to be given 21 days apart. Those two vaccines are to be administered 28 days apart.
COVID-19 vaccines will be offered to Canadians free of charge, and will not be mandatory.
“It's an exceptional day for Canada. It's one step along the road, it's one tool in terms of our fight against COVID-19, along with all of the other measures," said Sharma. "We have other vaccines that will be likely coming as well, but I think in a year where we haven't had a lot of good news, this is a bit of good news. And I think we should take a moment to acknowledge that, and then we're all going to get back to work."