New insights on the genetic drivers behind a rare type of fatal pediatric brain cancer may lead to the development of new patient-targeted treatments, a new study suggests.
Each year about 30 children in Canada are diagnosed with diffuse intrinsic pontine glioma (DIPG), a pediatric cancer for which there is no effective treatment.
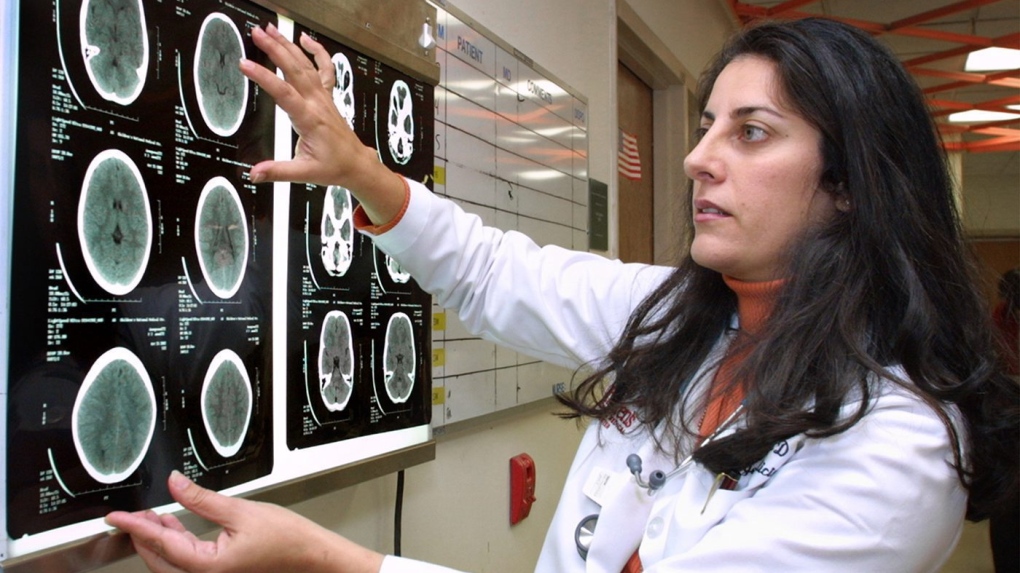
Because DIPG tumours occur in the middle of the brainstem – specifically in the "pons" region that controls vital functions such as breathing, heart regulation and movement – doctors are not able to surgically remove them.
As well, biopsies are rarely performed on the tumours, meaning researchers have faced challenges investigating the genetic landscape of DIPG.
But in a new study from Toronto's Hospital for Sick Children, researchers have uncovered the genetic drivers of DIPG.
The study, published online Sunday in the journal Nature Genetics, found that DIPG is comprised of three molecularly distinct subgroups: "MYCN," "silent" and "H3-K27M."
"Although previously considered to be one disease, DIPG represents three distinct subgroups with different methylation, expression, copy number alteration (CAN) and mutational profiles," the authors write.
The results from the study show that DIPG tumours are distinct from adult brain cancer, the study says.
Dr. Cynthia Hawkins, a neuropathologist and the study's principal investigator, said the discovery will have a significant impact on the development of DIPG treatment options.
"This work gives us the opportunity to make some real progress for these patients and their families," she said in a statement.
"We found that there are actually different genes driving the behaviour of each DIPG subgroup, which has key implications for the design of appropriate and more targeted therapy for these tumours. Put simply, it means that each subgroup will require a different therapy."
During the course of the study, researchers performed detailed genetic analysis of more than 60 DIPG samples obtained from Canada, the U.S., Australia and the U.K.
Hawkins said the team was surprised by how frequently mutations in the cancer occurred.
"Most of the time when you do genetic studies, you may find mutations in five per cent of the cases. Here we found different targetable genes in 20 to 80 per cent of the cases," she said.
"This was unexpected and very encouraging because from a clinical trial perspective, this could mean we have the opportunity to have a greater impact on more patients."
The team says additional research into DIPG is already underway, with researchers looking at the genetic mutation specific to each subgroup, as well as examining which targeted therapies may work.
Targeted drug therapies are particularly important for patients with inoperable tumours.
"With other cancers, surgeons aim to remove the majority of the tumour and follow up with radiation and/or chemotherapy to attack the remaining cancer," Hawkins said. "For DIPG, in order to make an impact, the medicine would need to treat the entire tumour."
Symptoms of DIPG first occur in patients between the ages of five and seven. As the tumours grow, they cause a buildup of pressure on the cranial nerves in the pons region of the brainstem. These nerves control muscles in the eyes, face and mouth.
As a result, symptoms of DIPG can include double vision, difficulty controlling eye movement, facial expression and chewing or swallowing. The tumour can also put pressure on other nerves that may cause weakness in the limbs, and difficulty speaking and walking.
To date, radiation therapy has been the main treatment used on the tumours, as surgery is not an option and chemotherapy has shown no benefit.
According to the DIPG Registry, fewer than 10 per cent of children with DIPG survive two years after diagnosis.