TORONTO -- A small clinical trial of the so-called "liberation treatment" for multiple sclerosis has found that the intervention did not improve patients' symptoms and in some cases even made their disease worse, researchers say.
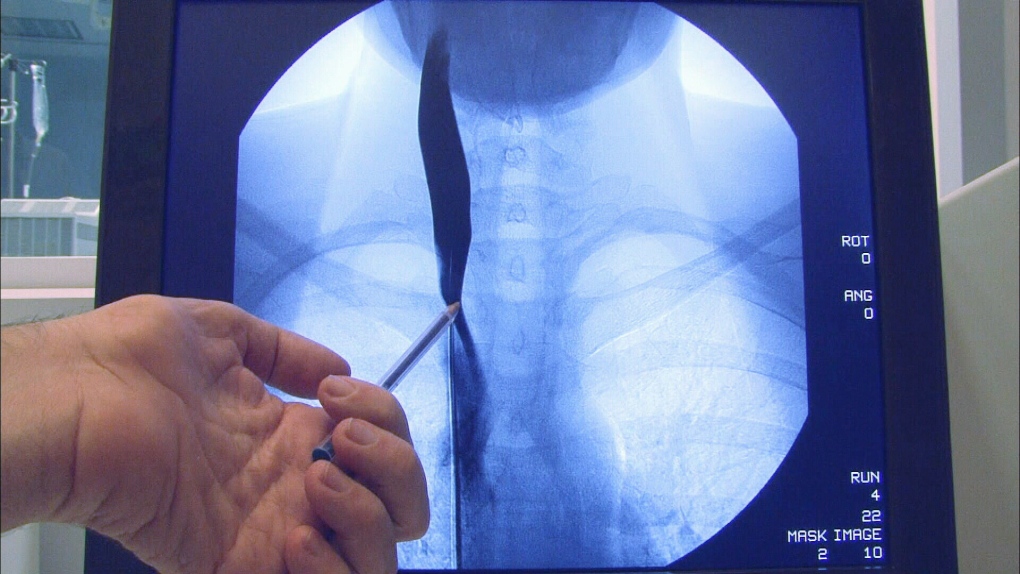
The University of Buffalo study of 30 MS patients found that the treatment -- which involves unblocking neck veins to improve blood drainage from the brain -- is safe. But the procedure had no benefit for patients' symptoms, disease progression or quality of life measures.
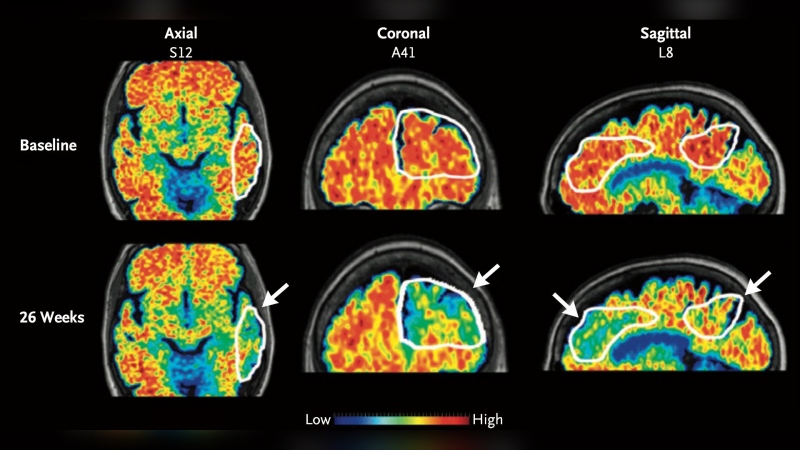
As well, MRI scans showed some patients had increased brain lesions, one of the hallmarks of the progressive neurological disease, after undergoing the vein-opening procedure, said neurosurgeon Dr. Adnan Siddiqui, co-principal investigator of the pilot study.
"What we found was rather surprising and unexpected," said Siddiqui. "It was quite the opposite of what we originally expected to find. The study showed that endovascular treatment of stenosed (blocked) veins had no effect in MS patients."
Italian vascular surgeon Paulo Zamboni proposed in 2009 that a condition he dubbed chronic cerebrospinal venous insufficiency, or CCSVI, might be a cause of MS.
Zamboni suggested that opening up neck and chest veins with balloon angioplasty, the same procedure used to unblock coronary arteries, could help relieve symptoms and might even stop progression of the disease.
Since then, an estimated 30,000 MS patients worldwide have sought the unproven treatment in clinics that have popped up in such countries as Poland, Bulgaria, India and Mexico, and to a lesser extent in the United States.
Included in those medical tourists are thousands of Canadians with MS: the experimental treatment is not offered in Canada, through a federally sponsored clinical trial is in the process of enrolling patients.
The Buffalo study set out to assess the safety of venous angioplasty for MS patients as well as its effectiveness by comparing subjects given the procedure and those who received a sham treatment.
The first 10 patients all got the procedure, then in a second phase of 20 more patients, half received venous angioplasty while the other 10 got the bogus therapy. None of the participants knew which treatment they were getting.
The findings suggest "that there's likely no benefit and possibly harm to venous angioplasty," Siddiqui said Friday from Buffalo.
"However, is this the last word on venous angioplasty? Absolutely not. I think a much larger cohort (group of patients) would be required to really demonstrate that definitively."
The researchers say MS patients should only consider having the procedure as part of a clinical trial, instead of in out-patient clinics that charge thousands of dollars for their services.
Even so, Siddiqui said the study does not negate the hypothesis that CCSVI may have a role to play in MS or other neurological diseases.
"That relationship remains extremely interesting and certainly we remain committed to evaluating that further and understanding that relationship further," he said.
"Our finding only suggests that using a balloon to open up observed venous narrowings in our cohort of patients, which was very carefully selected, did not show any benefit and demonstrated possible harm when looking at MRI activity."
Perhaps using balloon angioplasty isn't the right treatment option, Siddiqui suggested.
"I'm convinced that this particular intervention does not work for this particular disease finding," he said.
"So what we'd like to do is take a step back and really understand the disease further, so we could come up with a much more directed hypothesis as to what kind of intervention will actually work for these patients."