TORONTO -- A hand sanitizer meant to protect people from germs is being recalled because of bacterial contamination, Health Canada said Thursday.
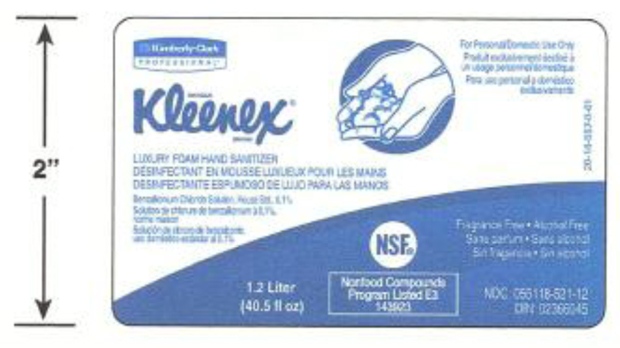
Kimberly-Clark is recalling its Kleenex-brand Luxury Foam Hand Sanitizer after company testing detected bacteria that may pose serious health risks to people with weakened immune systems, especially those with the lung disorder cystic fibrosis.
The bacteria identified in the tested samples are from the Burkholderia cepacia group. These bacteria pose little risk to healthy people, but for those with immune systems weakened by other illnesses, the microbes can cause serious problems, including pneumonia and blood infection.
The affected hand sanitizer comes in one-litre and 1.2-litre containers, and is used in large-volume dispensers, such as those found in public areas and workspaces.
The recall affects about 430 containers, which were distributed to retail stores and wholesalers across Canada.
Health Canada said companies or individuals who have purchased the affected product should remove it from use.
Consumers with compromised immune systems should not use the affected Kleenex sanitizer or any sanitizing product that can't be identified from its dispenser.
Health Canada said consumers should speak to their health-care practitioner about any questions or concerns regarding the product.
The affected products are:
-- Kleenex Luxury Foam Hand Sanitizer (Benzalkonium Chloride, 0.1%), 1,000 ml manual cassette (used in manual dispensers); Drug Identification Number: 02366045; lot number SA1229ANB.
--Kleenex Luxury Foam Hand Sanitizer (Benzalkonium Chloride, 0.1%), 1,200 ml E-Cassette (used in electronic dispensers); Drug Identification Number: 02366045; lot number SA1229ANA.
Additional information about the recall can be obtained by calling Kimberly-Clark Professional Corp. toll-free at 1-888-346-4652.
Any adverse health reaction linked to the product can be reported to Health Canada by calling toll-free at 1-866-234-2345.