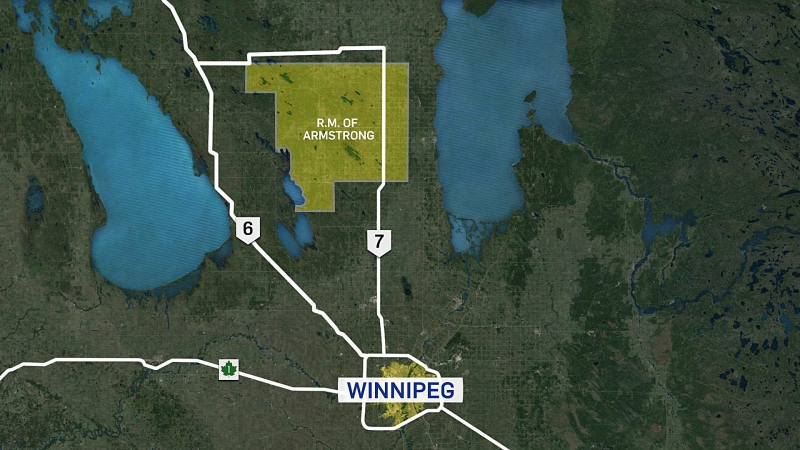
Toronto -
Health Canada approval for COVID-19 vaccines for babies and toddlers could take place in early 2022, Chief Medical Officer of Health Dr. Theresa Tam said on Friday.
"Pfizer and Moderna are continuing to complete their clinical trials for that youngest age group, they believe that the earliest I think that we will see vaccines for that age group will be in the new year," Tam told reporters.
So far, Tam said Health Canada has not received any submissions for vaccines for the youngest age group. However, Pfizer's data could be available by the end of this year.
“Which still means that we will not be implementing this program, I don’t think, until early 2022," Tam said.
As for Moderna, the company is currently testing a version of their COVID-19 vaccine with only a 25-microgram dose, compared to the 50 micrograms for children ages six to 11 and far smaller than the 100-microgram dose already approved for teens and adults.
There are a handful of Moderna trials underway in Canada, and more than 80 in the United States.
Pfizer's vaccine for children five to 11 years of age was approved by Health Canada last week and the first shipment arrived in Canada on Sunday. Moderna's under-12 vaccine is still being reviewed by the regulator.
With files from CTV News Montreal