
American millionaire Jonathan Lehrer denied bail after being charged with killing Canadian couple
American millionaire Jonathan Lehrer, one of two men charged in the killings of a Canadian couple in Dominica, has been denied bail.
BioNTech's co-founder and top executive said the vaccine maker has no plans to enforce its intellectual property rights should organizations in Africa strike out on their own to produce unauthorized versions of the company's shot.
"Our goal is not to keep others from using our technology. Our goal is rather to actively see to it that our technology is available on all continents as safely and as widely as possible," CEO Ugur Sahin told Reuters TV on Wednesday when asked whether he would pursue breaches of patents or patents pending in Africa.
The remarks come after the company, which developed the western world's most widely used COVID-19 shot with U.S. drugmaker Pfizer mapped out a plan to enable African countries to produce its Comirnaty-branded shot under BioNTech's supervision.
Sahin said the proprietary offshore production venture, dubbed Biontainer, was superior to hands-off data sharing because of the many pitfalls in quality maintenance.
At an earlier news conference Sahin said that novel cancer therapies BioNTech is working on will in future be made available on the continent at affordable prices.
Sahin's pledge on property rights echoes a similar statement by Moderna, which in 2020 said it would not enforce patents related to COVID-19 vaccines during the pandemic.
Different efforts are afoot to ease Africa's reliance on vaccine imports amid heated debate over whether Western vaccine pioneers are doing enough to support that cause.
Afrigen Biologics of South Africa, working with the World Health Organization, has set out to produce a version of Moderna's COVID-19 shot, even though it has not managed to win the U.S. vaccine developer's assistance.
Separately, South African-American businessman Patrick Soon-Shiong opened a vaccine plant in Cape Town in January, intended to help his NantSA company make COVID-19 shots in future.
Sahin, for his part, said BioNTech's venture to deliver a vaccine factory to Africa in the form of an assembly kit was the fastest contribution it could make towards African self-reliance.
"This turnkey solution is really the best we can currently do from our point of view if we want to establish production in Africa fast," the CEO said.
WHO Director-General Tedros Adhanom Ghebreyesus welcomed BioNTech’s initiative as a complement to WHO’s technology transfer hub involving Afrigen.
Speaking to Reuters TV, Sahin said its production line would comply with U.S. and European manufacturing standards.
A treaty establishing a regulator for the continent, the African Medicines Agency, came into force in November but the institution has yet to take shape.
While BioNTech's future African production line is designed to also make shots against malaria or tuberculosis, the most likely first product would be against COVID-19.
"That's the blueprint we will likely transfer first," BioNTech's Sahin told Reuters.
While the facility, dubbed Biontainer, will initially be run by BioNTech staff, the company plans to train local partners to take over the complex vaccine-making procedure that the company says involves 50,000 steps to complete.

American millionaire Jonathan Lehrer, one of two men charged in the killings of a Canadian couple in Dominica, has been denied bail.
Cabinet minister Dominic LeBlanc says he plans to run in the next election as a candidate under Prime Minister Justin Trudeau's leadership, amid questions about his rumoured interest in succeeding his longtime friend for the top job.
A male columnist has apologized for a cringeworthy moment during former University of Iowa superstar and college basketball's highest scorer Caitlin Clark's first news conference as an Indiana Fever player.
The United States has vetoed a widely backed UN resolution that would have paved the way for full United Nations membership for the state of Palestine.
A group of suspects that allegedly defrauded seniors across Ontario and other parts of Canada using a so-called emergency grandparent scam appear to have ties to 'Italian traditional organized crime,' according to an investigator involved in the OPP-led probe.
Health Canada will change its longstanding policy restricting gay and bisexual men from donating to sperm banks in Canada, CTV News has learned. The federal health agency has adopted a revised directive removing the ban on gay, bisexual and other men who have sex with men, effective May 8.
Prince Harry, the son of King Charles III and fifth in line to the British throne, has formally confirmed he is now a U.S. resident.
Kevin the cat has been reunited with his family after enduring a harrowing three-day ordeal while lost at Toronto Pearson International Airport earlier this week.
Technology from the 19th century has been brought out of retirement at a Newfoundland gardening store, as staff look for all the help they can get to fill orders during a busy season.
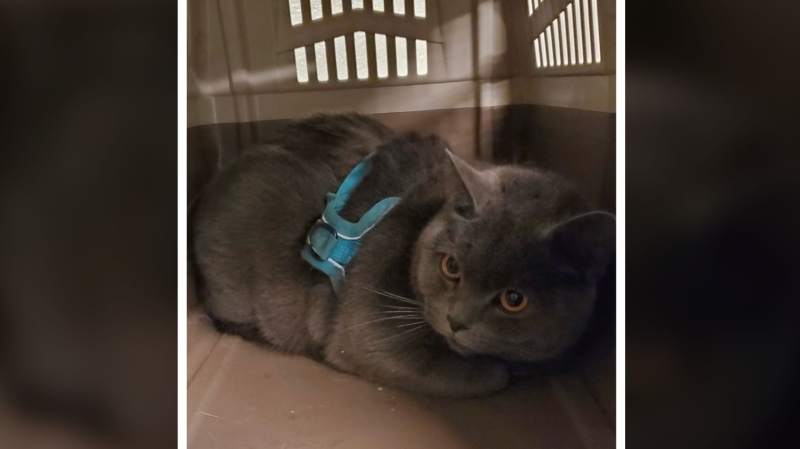
Kevin the cat has been reunited with his family after enduring a harrowing three-day ordeal while lost at Toronto Pearson International Airport earlier this week.
Molly Knight, a grade four student in Nova Scotia, noticed her school library did not have many books on female athletes, so she started her own book drive in hopes of changing that.
Almost 7,000 bars of pure gold were stolen from Pearson International Airport exactly one year ago during an elaborate heist, but so far only a tiny fraction of that stolen loot has been found.
When Les Robertson was walking home from the gym in North Vancouver's Lower Lonsdale neighbourhood three weeks ago, he did a double take. Standing near a burrow it had dug in a vacant lot near East 1st Street and St. Georges Avenue was a yellow-bellied marmot.
A moulting seal who was relocated after drawing daily crowds of onlookers in Greater Victoria has made a surprise return, after what officials described as an 'astonishing' six-day journey.
Just steps from Parliament Hill is a barber shop that for the last 100 years has catered to everyone from prime ministers to tourists.
A high score on a Foo Fighters pinball machine has Edmonton player Dave Formenti on a high.
A compound used to treat sour gas that's been linked to fertility issues in cattle has been found throughout groundwater in the Prairies, according to a new study.
While many people choose to keep their medical appointments private, four longtime friends decided to undergo vasectomies as a group in B.C.'s Lower Mainland.