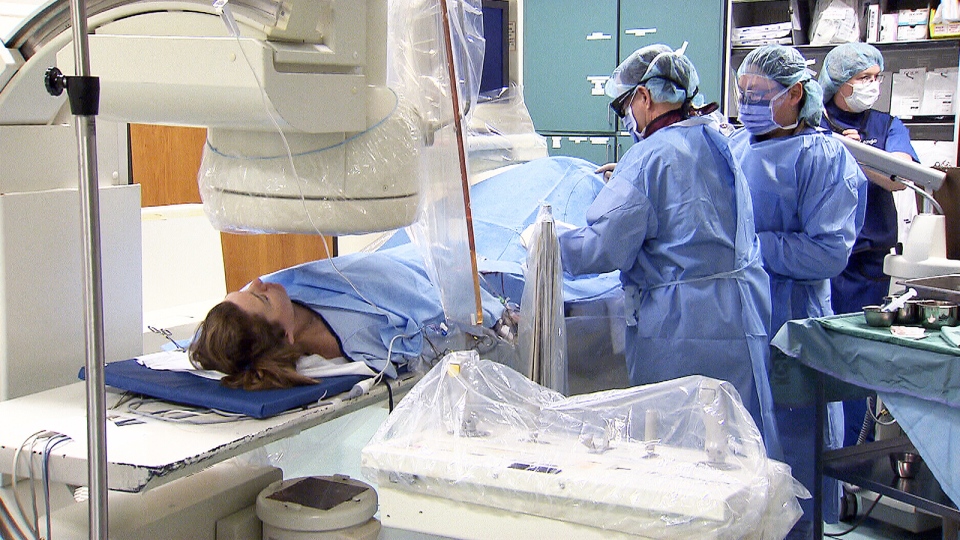
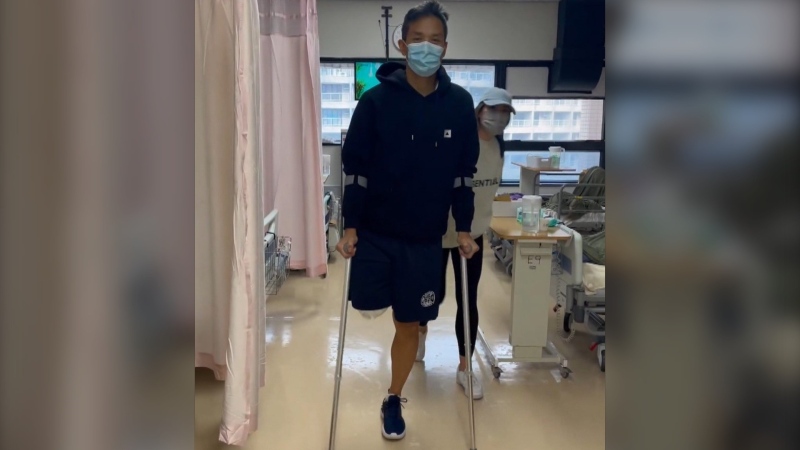
Canadians who were among multiple sclerosis sufferers waiting to take part in a clinical trial into the so-called liberation treatment were disappointed on Monday by news that the trial was cancelled.
The Saskatchewan government was told that the Albany Medical Centre in New York has stopped its trial into the effectiveness of angioplasty in treating CCSVI (chronic cerebro-spinal venous insufficiency) and relieving symptoms of multiple sclerosis. The treatment involves opening blocked neck veins.
There were 86 planned participants from Saskatchewan -- a province home to one of the highest rates of M.S. sufferers compared to the rest of Canada. The province had committed up to $2.2 million to have its patients participate in the Albany study.
Clinical trial lead Dr. Gary Siskin told Saskatchewan’s Ministry of Health that he wasn’t able to meet the overall target enrolment needed for the study to meet U.S. government requirements for a clinical trial.
Siskin told CTV News that he’s “very disappointed” the trial had to be cancelled.
He spoke about the difficulty in getting people to enrol in the study, as participants seemed reluctant because of the 50/50 chance that they would receive a placebo treatment instead of the real thing.
Siskin, an interventional radiologist, has treated several hundred patients outside of the trial -- but he stopped in favour of doing formal research into the treatment. His was the only FDA-approved study for CCSVI therapy.
He says despite the setback, his interest in CCSVI is undeterred. "This in no way diminishes my interest in CCSVI," he said.
He told CTV News that in his opinion, narrowed veins in patients with symptoms of MS "is a diagnostic entity” and is “real.”
"I've seen too many people who have responded to treatment to say they are making it up," Siskin said.
Saskatchewan Health Minister Dustin Duncan also expressed disappointment at the news for the approximately 3,500 MS sufferers in his province.
“Our government wants to do everything it can to search for answers and further the science for people with MS,” said Duncan. “That’s why Saskatchewan was supportive of this trial and will continue to be supportive of research that may provide answers for those with MS and their families.”
With files from CTV's medical specialist Avis Favaro