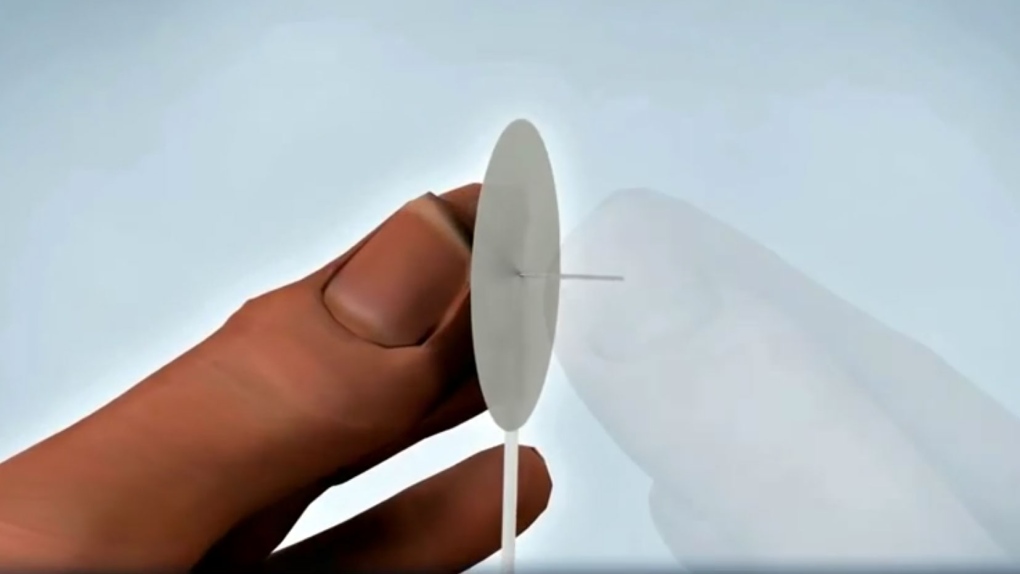
TORONTO - Health Canada is warning of a potential safety issue regarding insulin pumps for diabetics distributed by Medtronic of Canada Inc., Roche Diagnostics, LifeScan Canada Inc., and Auto Control Medical Inc.
The manufacturer Unomedical says there has been an increase in reports of the tubing becoming detached at the connection site for the pumps.
The company says that if the tubing becomes detached, insulin delivery would be interrupted and the alarm on the device would not notify the user.
An interruption in insulin delivery could result in elevated blood sugar levels in a patients, which could lead to serious and potentially fatal diabetic ketoacidosis. Symptoms of diabetic ketoacidosis may include nausea, vomiting, shortness of breath and excess thirst and urination.
The manufacturer says patients should continue to use their pump, but check the tubing at the connector site to ensure it is not loose and to frequently monitor blood sugar levels.
Unomedical says that if the tubing has become detached, patients should not try to re-attach it themselves but immediately replace the infusion set.