TORONTO -- At less than four weeks old, little Zain Rajani has no idea he's made history.
He's the world's first baby born following an innovative procedure being hailed as a game-changer in the treatment of infertility.
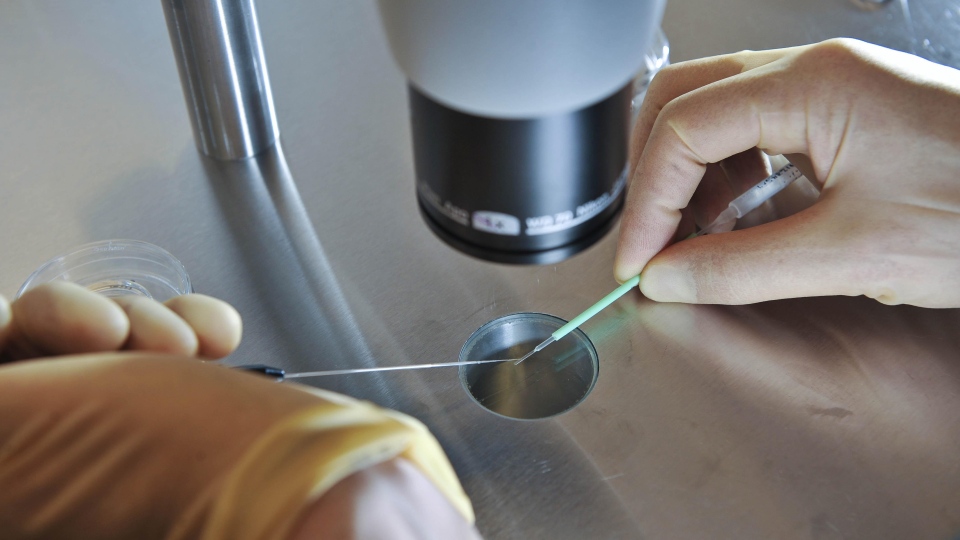
The Toronto infant was born on April 13 through in-vitro fertilization. Eggs extracted from his mother Natasha Rajani were given a power boost using parts of the cell called the mitochondria, which convert nutrients into energy and are known as the powerhouses of every cell.
First, mitochondria were taken from egg precursor cells in the lining of her ovaries. These cells are the yet-to-be developed forerunners of fully formed eggs.
The mitochondria were then injected into her eggs along with her husband's sperm. Resulting embryos were then implanted in her uterus.
Rajani, 34, is among nine Toronto women with poor egg quality who have been able to get pregnant after going through the Augment procedure.
The eight remaining women -- including one carrying twins -- are expected to give birth in the coming weeks and months.
"We're extremely grateful for having been able to take part in the procedure and as a result exceptionally grateful to have Zain," said Rajani, who hadn't thought of her infant as being a pioneer until a friend pointed out that Zain had made history.
"I always thought, and I still to this day see Zain as a symbol of hope for other women suffering with infertility, especially as it relates to poor egg quality," she said Friday.
Rajani and her husband Omar had been trying to start a family for about four years, but following an ectopic pregnancy and a failed IVF attempt, the couple wasn't sure what to try next. Despite her relatively young age, doctors had determined Rajani's eggs were not robust enough to produce healthy embryos.
But that changed with Augment, a technique developed by OvaScience, a Cambridge, Mass.-based fertility technology company, which has been working with doctors at Mount Sinai Hospital to introduce the treatment. (About 150 women around the world have now had the procedure, which is not yet approved in the U.S.)
Dr. Robert Casper, medical director of Mount Sinai's Toronto Centre for Advanced Reproductive Technology (TCART), said 35 Toronto women aged 40 and under had the egg-recharging procedure, resulting in embryo transfers for 28 of the women. Fifteen women got pregnant, six subsequently miscarried.
"That's a pretty standard rate for the older infertility population," said Casper, noting that women's eggs can lose potency with age.
He likened the eggs waiting to mature inside the ovaries to a flashlight sitting on a shelf for many years.
"The flashlight itself is OK, but the batteries tend to run down over time," he said.
"What we're doing with this mitochondria injection is sort of replacing or adding more batteries to the eggs, to boost the energy up to be able to get that embryo to develop all the way to Day 5, when it will start to make its own new mitochondria."
Because mitochondria from egg precursor cells contain DNA identical to those in mature eggs, the procedure does not change the baby's genome. Using mitochondria from a donor egg in effect gives a child three biological parents.
"The cells are not manipulated in any way.... We're not changing the genetic makeup of the child at all," said Casper, who firmly believes the technique is safe.
Having the procedure adds US$23,500 to the cost of IVF, which carries a price tag at TCART of $8,000 per cycle, although it can vary depending on the fertility clinic.
While not a "miracle" treatment, Casper said, it does boost some women's fertility, giving them a pregnancy rate of about 30 to 40 per cent, the standard success rate with IVF.
But even more exciting is what else might be achieved with the egg precursor cells, which were discovered in the ovaries of mice in 2004 by Harvard Medical School researcher Jonathan Tilly, and then subsequently found in humans.
"Essentially, those egg precursor cells can mature into eggs," said Michelle Dipp, co-founder and CEO of OvaScience.
The company is now developing a technique to grow up the precursor cells into mature eggs by moving them from the lining to the centre, or cortex, of the ovaries.
"We're working on that now and so we will be introducing that into patients this year," she said from Boston.
Women are born with a set number of eggs and once they're gone, fertility ends. But if the new technique proves successful, egg precursor cells could conceivably stop the ticking of the biological clock -- potentially allowing older women to get pregnant and give birth to their own biological child.
"Women get pregnant later in life with donor eggs all the time," said Casper, whose centre will be involved with the new research.
"That's why this seems to be what I consider a ground-breaking technique, because it does really change the paradigm."
As for the Rajanis, they banked two frozen embryos created at the same time as the one that grew into Zain, which they hope may one day mean a sibling or two for their new son.
"I think both of us feel like we're on Cloud 9," said Omar Rajani, 39.
"It's been a long journey for us, but we remained pretty hopeful throughout the whole process and we feel really fortunate to have received the treatment that helped us produce little Zain."