TORONTO -- Health Canada has secured a new supply of tuberculosis vaccine, filling a void left when the country's sole supplier recalled all its product earlier this year.
The department says it has entered a 19-month contract with Japan BCG Laboratory of Tokyo to buy TB vaccine.
The vaccine was given expedited approval, which was necessary because it was not previously licensed for use in Canada.
Canada hasn't had a supplier of TB vaccine since mid-June, when Sanofi Pasteur recalled 47,000 doses of TB vaccine because of fears it might have been contaminated.
The building where the vaccine was made was flooded last fall; Health Canada inspectors later found mould in what should have been a sterile manufacturing area.
Sanofi is working to repair the facility, which is in Toronto, but it doesn't expect to have product back on the market until the end of 2013.
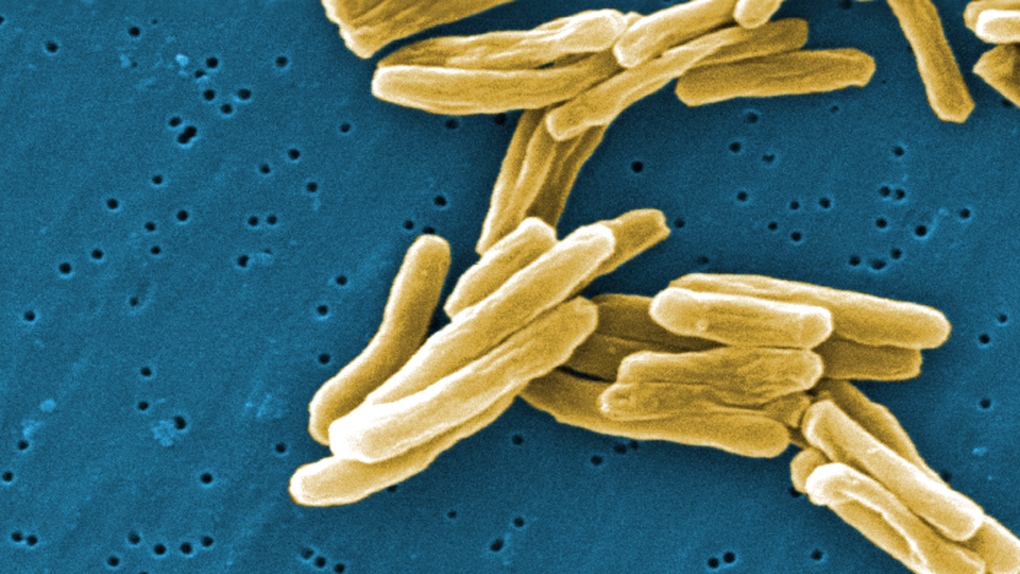
Tuberculosis vaccine is known as BCG vaccine, named after Albert Calmette and Camille Guerin who developed the strain used in it back in the early 1900s.
The vaccine uses a live but weakened strain of Mycobacterium bovis, the bacterium that causes tuberculosis in cows. It is related to the bacterium that causes TB in people.
BCG vaccine is not widely used in Canada. It is mainly employed to protect young children living in First Nations and Inuit communities where the risk of TB outbreaks is high.
A statement from Health Canada suggests it may be awhile still before the vaccine is available for administration.
"We expect that the BCG vaccine will be made available to the communities that need it in the coming months," the department says.
When it became apparent the country's sole supplier would be unable to fill orders for at least a year-and-a half, the Public Health Agency of Canada reached out to five other manufacturers which produce BCG vaccine outside the country.
Japan BCG Laboratory's product was chosen because of both the product's safety record and the fact the company is able to supply vaccine quickly, the Health Canada release says.