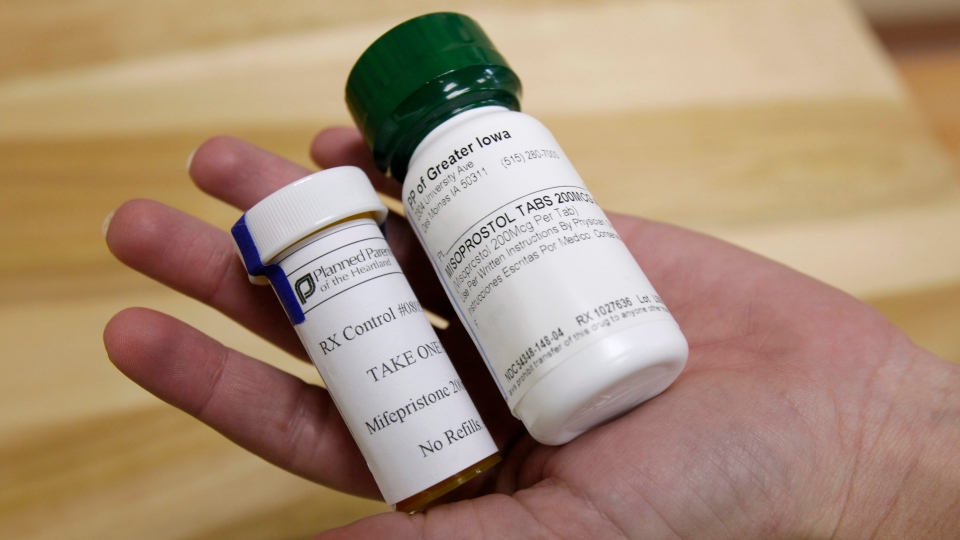
Health Canada has pushed back a decision on whether to approve the abortion drug mifepristone, which was slated for last month.
The Health agency has been looking at the application to approve the drug, also known as RU-486, since 2012. The tablet, which was developed in the 1980s, is taken to end an early pregnancy.
It works by blocking the hormone progesterone, and triggers a spontaneous abortion. Progesterone is necessary to maintain a pregnancy.
The World Health Organization has placed it on the list of "essential medication," and it is approved for use in some 60 countries, including the United States.
In Canada, doctors say its approval is critical, particularly for women in remote areas who don't have access to surgical abortions.
"I am very confident that (mifepristone) is a safe and effective medication for women … and that it will be less complex and dangerous for them than continuing an unwanted pregnancy," Dr. Wendy Norman, chair of the section of researchers at the College of Family Physicians of Canada, said in an interview with CTV News.
Since the drug's approval in the U.S. in 2000, there have been reports of serious bacterial infections and very rare cases of fatal septic shock. However, clinical trials by the U.S. Food and Drug Administration showed that there is "no causal relationship" between the incidents and use of the drug.
The FDA study found that the most common side effects included abdominal pain, nausea, headache, vomiting, diarrhea, dizziness and fatigue.
Despite this, anti-abortion groups are satisfied that the drug has yet to gain approval in Canada, and have campaigned to keep it off the market.
"I think that (Health Canada has) delayed it because there is a lot of controversy surround the drug – it is a lethal drug designed to kill," said Alissa Golob, a spokeswoman for the Campaign Life Coalition.
"I certainly hope we have helped slow down the decision because … we should not bring RU-486 into Canada," Golob added.
But Vicki Saporta, president of the National Abortion Federation Canada, says the delay is simply forcing desperate women to look for alternative ways of obtaining the drug.
"Some Canadian women have gone to our member clinics saying they purchased what they thought was mifepristone online,” Saporta said. “It was not effective and they have gone to obtain a surgical procedure."
Health Canada hasn't provided any further details on when it will issue a final decision.
With a report from CTV NewsMedical Specialist Avis Favaro and producer Elizabeth St. Philip