An elite group of influenza scientists has announced it wants to do controversial gain-of-function research on the new H7N9 bird flu virus that emerged this spring in China.
Their proposal, which is likely to reignite the heated and protracted debate that raged over the publication of gain-of-function studies on H5N1 bird flu, was outlined in a letter published simultaneously Wednesday by two top journals, Nature and Science.
The journals also published a letter from senior officials of the U.S. Department of Health and Human Services announcing it will require prior review of proposals for some gain-of-function studies on H7N9 viruses, if the work is being done with U.S. funding. A similar system is now in place for H5N1 gain-of-function studies.
Dutch virologist Ron Fouchier, who is one of the authors, said the idea behind the scientists' letter is for researchers to be transparent about what they plan to do in the hopes of fending off some of the concerns that emerged in the H5N1 controversy.
"One of the major accusations during the H5N1 controversy was that the flu community was not transparent, upfront about which experiments were done, why they where done, and how they were done safely. And so we are trying to prevent that accusation ... this time around," Fouchier, a global leader in this type of research, said in an interview.
Gain-of-function research involves adding mutations of a virus to see if it can acquire features it doesn't currently have, such as the ability to resist flu drugs or spread easily from person to person. The goal is to identify changes that would give a virus like H7N9 these capacities so that public health laboratories could be on the lookout for viruses with these changes that emerge in nature.
The virus, first spotted in late March, has to date led to 134 confirmed human cases, 43 of whom died.
Objections to the proposed research agenda were quick to materialize, with one opponent of gain-of-function work likening the letter to a manifesto issued without consultation with flu scientists elsewhere or the larger scientific community.
Simon Wain-Hobson, a professor of virology at the Pasteur Institute in Paris, also bristled at what he saw as an implied suggestion that gain-of-function work is now an accepted and integral step in understanding a new flu virus.
"This is just pure lobbying. It's hard-nosed lobbying. And I don't understand why Nature and Science are doing this," Wain-Hobson said. "I'm astounded by it."
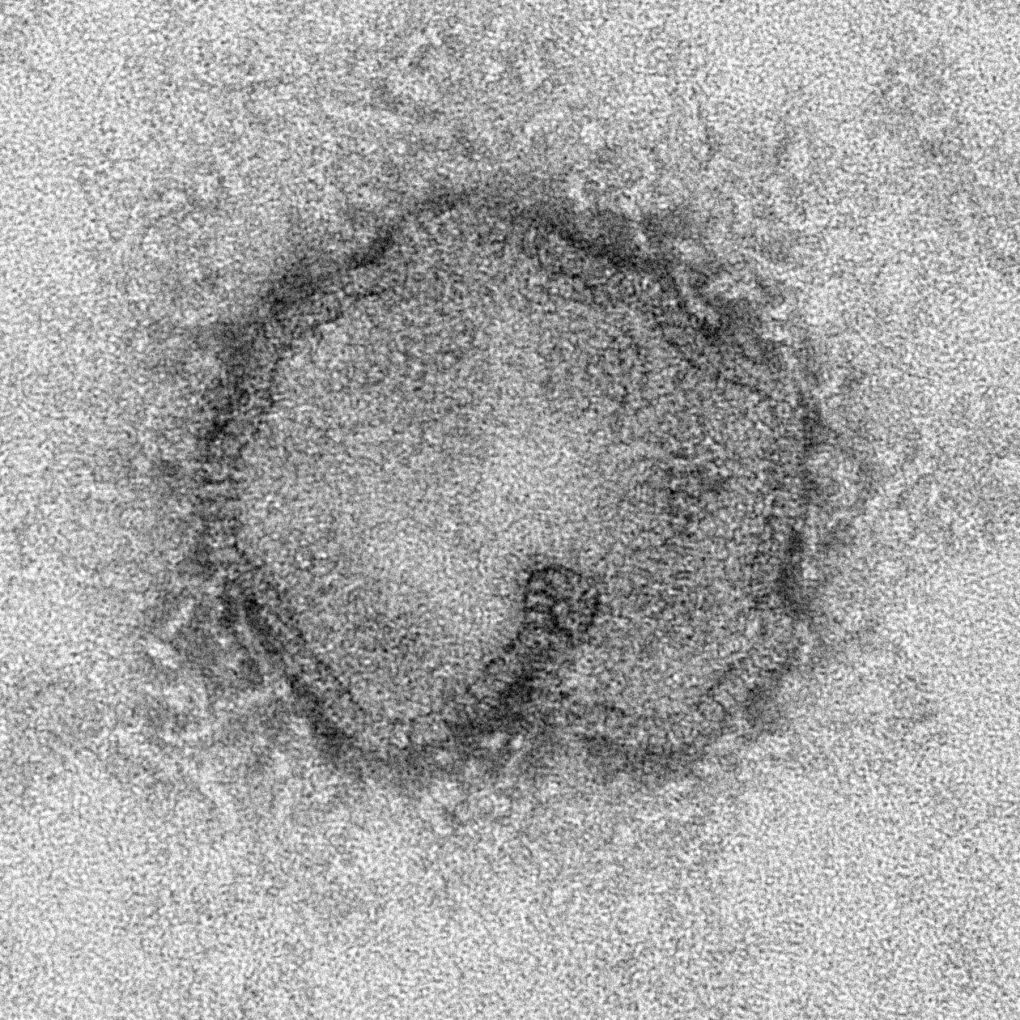
Fouchier said one of the studies he wants to do is to add a mutation in the receptor binding area of the H7N9 hemagglutinin, the protein that latches onto the cells in a host when a flu virus infects a person, a duck or some other animal.
Bird viruses bind to different cell receptors than human flu viruses, which is why people are rarely infected with bird flu viruses. But H7N9 has one mutation at the receptor binding site that seems to allow it to bind more easily to human cells. Past experience suggests one additional mutation at that location would make the virus more transmissible among people.
But engineering dangerous viruses to make them more so unsettles many scientists and some experts in the security community as well.
When Fouchier and another top flu researcher, Yoshihiro Kawaoka of the University of Wisconsin-Madison, tried in 2011 to publish studies outlining how one could make H5N1 viruses transmit among mammals, a U.S. expert panel initially advised that the papers should be published in abbreviated form only.
The National Science Advisory Board for Biosecurity -- or NSABB, as it is commonly called -- argued that by listing the mutations needed to make H5N1 transmit like seasonal flu viruses the researchers would be publishing a recipe for making a bird flu virus that could spread easily from person to person. They said that could lead to a situation where a lab that didn't have the needed containment facilities might produce a mutated virus and accidentally release it.
Michael Osterholm, director of the University of Minnesota's Center for Infectious Diseases Research and Policy is on the NSABB and was one of those who objected to full publication of the H5N1 gain-of-function studies. While the NSABB eventually withdrew its objection to publication of those studies, Osterholm remains concerned about the possibility of an accidental release of a mutated bird flu virus.
"I continue to support gain-of-function work from a basic research standpoint. But ... I sit in the middle here where I think there still are major questions about the risks-benefits of this work that need to be addressed," he said.
Osterholm is also director of the Minnesota Center of Excellence for Influenza Research and Surveillance, which is part of a network known as the Centers of Excellence for Influenza Research and Surveillance that is funded by the U.S. National Institute for Allergy and Infectious Diseases.
The scientists who wrote the H7N9 letter are funded through the network. Most are based in the United States, but a few, like Fouchier, do their work overseas with American funding.
Though Osterholm heads a network centre, he did not sign the letter. Among the reasons he gave is the fact that he thinks gain-of-function proponents oversell the benefits of the work when they argue it is critical for surveillance for bird flu viruses with pandemic potential and for design of better antiviral drugs and vaccines.
He said more than a year after Fouchier and Kawaoka's H5N1 studies were finally published, the global surveillance system for flu still doesn't have the capacity to rapidly sequence bird flu viruses to look for the mutations the research suggested could allow that virus to start spreading from person to person.
"It hasn't happened. Why should we believe it's going to happen with H7N9?" Osterholm asked.
In a related development on Wednesday, Fouchier published a study in Nature looking at whether H7N9 viruses can transmit via aerosols -- tiny virus-laced droplets spewed through coughing and sneezing -- in ferrets. The animals are considered the best model for predicting how flu viruses act in humans.
Several other groups have already done similar work, showing H7N9 does sometimes transmit from infected ferrets to healthy ones housed in nearby cages. In this study, three of four healthy ferrets caught the flu from the deliberately infected animals.
But Fouchier's lab went a step further. His team took a virus from one of the ferrets that caught the virus, and used it to infect four more animals. When four healthy animals were placed in a cage near the second group of deliberately infected ferrets, only one of the four healthy animals contracted the virus.
Fouchier says this suggests that rather than becoming more transmissible with generations of spread through ferrets -- which is what happens with H5N1 viruses -- H7N9 seems to become less transmissible. "That might be good news. (But) we don't know," he said.